Molecular basis for peptidoglycan recognition by a bactericidal lectin
- PMID: 20382864
- PMCID: PMC2867859
- DOI: 10.1073/pnas.0909449107
Molecular basis for peptidoglycan recognition by a bactericidal lectin
Abstract
RegIII proteins are secreted C-type lectins that kill Gram-positive bacteria and play a vital role in antimicrobial protection of the mammalian gut. RegIII proteins bind their bacterial targets via interactions with cell wall peptidoglycan but lack the canonical sequences that support calcium-dependent carbohydrate binding in other C-type lectins. Here, we use NMR spectroscopy to determine the molecular basis for peptidoglycan recognition by HIP/PAP, a human RegIII lectin. We show that HIP/PAP recognizes the peptidoglycan carbohydrate backbone in a calcium-independent manner via a conserved "EPN" motif that is critical for bacterial killing. While EPN sequences govern calcium-dependent carbohydrate recognition in other C-type lectins, the unusual location and calcium-independent functionality of the HIP/PAP EPN motif suggest that this sequence is a versatile functional module that can support both calcium-dependent and calcium-independent carbohydrate binding. Further, we show HIP/PAP binding affinity for carbohydrate ligands depends on carbohydrate chain length, supporting a binding model in which HIP/PAP molecules "bind and jump" along the extended polysaccharide chains of peptidoglycan, reducing dissociation rates and increasing binding affinity. We propose that dynamic recognition of highly clustered carbohydrate epitopes in native peptidoglycan is an essential mechanism governing high-affinity interactions between HIP/PAP and the bacterial cell wall.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

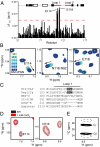
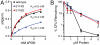
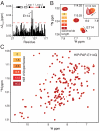


References
-
- Drickamer K. C-type lectin-like domains. Curr Opin Struct Biol. 1999;9:585–590. - PubMed
-
- Christa L, et al. HIP/PAP is an adhesive protein expressed in hepatocarcinoma, normal Paneth, and pancreatic cells. Am J Physiol. 1996;271:G993–1002. - PubMed
-
- Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature. 1992;360:183–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

