Real-time visualization of complexin during single exocytic events
- PMID: 20383135
- PMCID: PMC2860691
- DOI: 10.1038/nn.2532
Real-time visualization of complexin during single exocytic events
Abstract
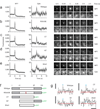
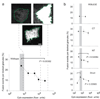
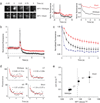
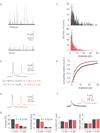
Understanding the fundamental role of soluble NSF attachment protein receptor (SNARE) complexes in membrane fusion requires knowledge of the spatiotemporal dynamics of their assembly. We visualized complexin (cplx), a cytosolic protein that binds assembled SNARE complexes, during single exocytic events in live cells. We found that cplx appeared briefly during full fusion. However, a truncated version of cplx containing only the SNARE-complex binding region persisted at fusion sites for seconds and caused fusion to be transient. Resealing pores with the mutant cplx only partially released transmitter and lipid probes, indicating that the pores are narrow and not purely lipidic in structure. Depletion of cplx similarly caused secretory cargo to be retained. These data suggest that cplx is recruited at a late step in exocytosis and modulates fusion pores composed of SNARE complexes.
Figures



References
-
- Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nature Rev. Mol. Cell Biol. 2006;7:631–643. - PubMed
-
- Weber T, et al. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. - PubMed
-
- Han X, Wang CT, Bai J, Chapman ER, Jackson MB. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. - PubMed
-
- Kesavan J, Borisovska M, Bruns D. v-SNARE actions during Ca2+-triggered exocytosis. Cell. 2007;131:351–363. - PubMed
-
- Jackson MB, Chapman ER. Fusion pores and fusion machines in Ca2+-triggered exocytosis. Annu. Rev. Biophys. Biomol. Struct. 2006;35:135–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

