Transcriptional response of Candida albicans biofilms following exposure to 2-amino-nonyl-6-methoxyl-tetralin muriate
- PMID: 20383169
- PMCID: PMC4002748
- DOI: 10.1038/aps.2010.33
Transcriptional response of Candida albicans biofilms following exposure to 2-amino-nonyl-6-methoxyl-tetralin muriate
Abstract
Aim: To identify changes in the gene expression profile of Candida albicans (C albicans) biofilms following exposed to 2-amino-nonyl-6-methoxyl-tetralin muriate(10b) and clarify the mechanism of 10b against C albicans biofilms.
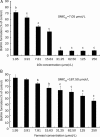
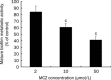
Methods: Anti-biofilm activity of 10b was assessed by tetrazolium (XTT) reduction assay and the action mechanism against biofilms was investigated by cDNA microarray analysis and real-time RT-PCR assay.
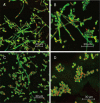
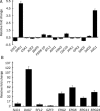
Results: Ten differentially expressed genes were directly linked to biofilm formation and filamentous or hyphal growth (eg, NRG1, ECE1 and CSA1). Decreased gene expression was involved in glycolysis (eg, HXK2 and PFK1) and antioxidant defense (eg, SOD5), while increased gene expression was associated with enzymes that specifically hydrolyzed beta-1,3 glucan (XOG1), and with lipid, fatty acid and sterol metabolism (eg, SLD1, ERG6 and ERG2). Functional analysis indicated that addition of anti-oxidant ascorbic acid reduced inhibitory efficiency of 10b on mature biofilm.
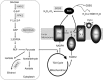
Conclusion: Inhibition of 10b on biofilm formation possibly depends on impairing the ability of C albicans to change its morphology via altering the expression of biofilm formation genes. Mitochondrial aerobic respiration shift and endogenous ROS augmentation might be a major contribution to reduce mature biofilm metabolic activity. The data may be useful for the development of new strategies to reduce the incidence of device-associated infections.
Figures

Similar articles
-
2-amino-nonyl-6-methoxyl-tetralin muriate activity against Candida albicans augments endogenous reactive oxygen species production --a microarray analysis study.FEBS J. 2011 Apr;278(7):1075-85. doi: 10.1111/j.1742-4658.2011.08021.x. Epub 2011 Feb 23. FEBS J. 2011. PMID: 21251230
-
2-Amino-nonyl-6-methoxyl-tetralin muriate inhibits sterol C-14 reductase in the ergosterol biosynthetic pathway.Acta Pharmacol Sin. 2009 Dec;30(12):1709-16. doi: 10.1038/aps.2009.157. Epub 2009 Nov 16. Acta Pharmacol Sin. 2009. PMID: 19915585 Free PMC article.
-
Development of Candida albicans Biofilms Is Diminished by Paeonia lactiflora via Obstruction of Cell Adhesion and Cell Lysis.J Microbiol Biotechnol. 2018 Mar 28;28(3):482-490. doi: 10.4014/jmb.1712.12041. J Microbiol Biotechnol. 2018. PMID: 29316739
-
Antifungal susceptibility of Candida albicans in biofilms.Mycoses. 2012 May;55(3):199-204. doi: 10.1111/j.1439-0507.2011.02076.x. Epub 2011 Jul 28. Mycoses. 2012. PMID: 21793943 Review.
-
Biofilm of Candida albicans: formation, regulation and resistance.J Appl Microbiol. 2021 Jul;131(1):11-22. doi: 10.1111/jam.14949. Epub 2020 Dec 9. J Appl Microbiol. 2021. PMID: 33249681 Review.
Cited by
-
Possible Molecular Targeting of Biofilm-Associated Genes by Nano-Ag in Candida albicans.Appl Biochem Biotechnol. 2024 Jul;196(7):4205-4233. doi: 10.1007/s12010-023-04758-6. Epub 2023 Nov 3. Appl Biochem Biotechnol. 2024. PMID: 37922031
References
-
- Sato T, Watanabe T, Mikami T, Matsumoto T. Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. Biol Pharm Bull. 2004;27:751–2. - PubMed
-
- Nett J, Ande D. Candida albicans biofilm development, modeling a host-pathogen interaction. Curr Opin Microbiol. 2006;9:340–5. - PubMed
-
- Oliveira M, Nunes SF, Carneiro C, Bexiga R, Bernardo F, Vilela CL. Time course of biofilm formation by Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol. 2007;124:187–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

