A chemical screen to identify novel inhibitors of fin regeneration in zebrafish
- PMID: 20384483
- PMCID: PMC2946367
- DOI: 10.1089/zeb.2009.0633
A chemical screen to identify novel inhibitors of fin regeneration in zebrafish
Abstract
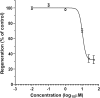
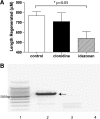
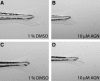
We performed a chemical screen to look for novel inhibitors of zebrafish caudal fin regeneration. In a pilot screen, 520 compounds were tested. Two compounds, budesonide and AGN192403, abrogated fin regeneration. One compound in particular, AGN192403, targets the imidazoline receptor, a pathway not previously linked to fin regeneration. In addition to inhibiting regeneration of the adult fin, AGN192403 also blocked regeneration of the larval fin fold. Finally, the inhibitory effect of AGN192403 on fin regeneration persisted after removal of the drug. These studies demonstrate that chemical screening is feasible in adult zebrafish and that it is a reasonable strategy to use for exploring the biology of regeneration.
Figures





References
-
- Brockes JP. Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. - PubMed
-
- Khan AZ. Mudan SS. Liver regeneration: mechanisms, mysteries and more. ANZ J Surg. 2007;77:9–14. - PubMed
-
- Becker T. Wullimann MF. Becker CG. Bernhardt RR. Schachner M. Axonal regrowth after spinal cord transection in adult zebrafish. J Comp Neurol. 1997;377:577–595. - PubMed
-
- Bereiter-Hahn J. Zylberberg L. Regeneration of teleost fish scale. Comp Biochem Physiol. 1993;105A:625–641.
-
- Bernhardt RR. Tongiorgi E. Anzini P. Schachner M. Increased expression of specific recognition molecules by retinal ganglion cells and by optic pathway glia accompanies the successful regeneration of retinal axons in adult zebrafish. J Comp Neurol. 1996;376:253–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

