B7 family molecules as regulators of the maternal immune system in pregnancy
- PMID: 20384620
- PMCID: PMC3426307
- DOI: 10.1111/j.1600-0897.2010.00841.x
B7 family molecules as regulators of the maternal immune system in pregnancy
Abstract
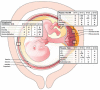
Placental and fetal growth and development are associated with chronic exposure of the maternal immune system to fetally derived, paternally inherited antigens. Because maternal lymphocytes are aware of fetal antigens, active tolerance mechanisms are required to ensure unperturbed progression of pregnancy and delivery of a healthy newborn. These mechanisms of tolerance may include deletion, receptor downregulation, and anergy of fetal antigen-specific cells in lymphoid tissues, as well as regulation at the maternal-fetal interface by a variety of locally expressed immunoregulatory molecules. The B7 family of costimulatory molecules comprises one group of immunoregulatory molecules present in the decidua and placenta. B7 family members mediate both inhibitory and stimulatory effects on T-cell activation and effector functions and may play a critical role in maintaining tolerance to the fetus. Here, we review the known functions of the B7 family proteins in pregnancy.
Figures


Similar articles
-
The Role of B7 Family Molecules in Maternal-Fetal Immunity.Front Immunol. 2020 Mar 24;11:458. doi: 10.3389/fimmu.2020.00458. eCollection 2020. Front Immunol. 2020. PMID: 32265918 Free PMC article. Review.
-
Immunoregulatory effects of lymphokine-driven placental cells on cloned T cells in vitro.Reg Immunol. 1988 Jul-Aug;1(1):69-77. Reg Immunol. 1988. PMID: 3275130
-
Adoptive transfer of paternal antigen-hyporesponsive T cells induces maternal tolerance to the allogeneic fetus in abortion-prone matings.J Immunol. 2004 Sep 15;173(6):3612-9. doi: 10.4049/jimmunol.173.6.3612. J Immunol. 2004. PMID: 15356105
-
Children's immunology, what can we learn from animal studies (1): Decidual cells induce specific immune system of feto-maternal interface.J Toxicol Sci. 2009;34 Suppl 2:SP331-9. doi: 10.2131/jts.34.sp331. J Toxicol Sci. 2009. PMID: 19571488 Review.
-
Immunological relationship between the mother and the fetus.Int Rev Immunol. 2002 Nov-Dec;21(6):471-95. doi: 10.1080/08830180215017. Int Rev Immunol. 2002. PMID: 12650238 Review.
Cited by
-
Maternal CD4⁺ and CD8⁺ T cell tolerance towards a fetal minor histocompatibility antigen in T cell receptor transgenic mice.Biol Reprod. 2013 Oct 31;89(4):102. doi: 10.1095/biolreprod.113.110445. Print 2013 Oct. Biol Reprod. 2013. PMID: 24025737 Free PMC article.
-
Fetal Growth Restriction and Subsequent Low Grade Fetal Inflammatory Response Are Associated with Early-Onset Neonatal Sepsis in the Context of Early Preterm Sterile Intrauterine Environment.J Clin Med. 2021 May 8;10(9):2018. doi: 10.3390/jcm10092018. J Clin Med. 2021. PMID: 34066888 Free PMC article.
-
Inflammation and pregnancy: the role of the immune system at the implantation site.Ann N Y Acad Sci. 2011 Mar;1221(1):80-7. doi: 10.1111/j.1749-6632.2010.05938.x. Ann N Y Acad Sci. 2011. PMID: 21401634 Free PMC article. Review.
-
Placental Galectins Are Key Players in Regulating the Maternal Adaptive Immune Response.Front Immunol. 2019 Jun 19;10:1240. doi: 10.3389/fimmu.2019.01240. eCollection 2019. Front Immunol. 2019. PMID: 31275299 Free PMC article.
-
The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin.Cell Transplant. 2018 Jan;27(1):31-44. doi: 10.1177/0963689717742819. Cell Transplant. 2018. PMID: 29562786 Free PMC article.
References
-
- Moldenhauer LM, Hayball JD, Robertson SA. Conceptus antigens activate the maternal immune response in pregnancy utilising maternal antigen presenting cells. J Immunol. 2009;71:148.
-
- Waldorf KM Adams, Yan Z, Stevens AM, Nelson JL. The changing maternal “self” hypothesis: a mechanism for maternal tolerance of the fetus. Placenta. 2007;28:378–382. - PubMed
-
- Jenkins MK, Pardoll DM, Mizuguchi J, Quill H, Schwartz RH. T-cell unresponsiveness in vivo and in vitro: fine specificity of induction and molecular characterization of the unresponsive state. Immunol Rev. 1987;95:113–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

