The realistic performance achievable with mycobacterial automated culture systems in high and low prevalence settings
- PMID: 20385000
- PMCID: PMC2862026
- DOI: 10.1186/1471-2334-10-93
The realistic performance achievable with mycobacterial automated culture systems in high and low prevalence settings
Abstract
Background: Diagnostic tests are generally used in situations with similar pre-test probability of disease to where they were developed. When these tests are applied in situations with very different pre-test probabilities of disease, it is informative to model the likely implications of known characteristics of test performance in the new situation. This is the case for automated Mycobacterium tuberculosis (MTB) liquid culture systems for tuberculosis case detection which were developed and are widely used in low burden settings but are only beginning to be applied on a large scale in high burden settings.
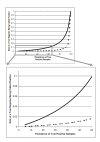
Methods: Here we model the performance of MTB liquid culture systems in high and low tuberculosis (TB) prevalence settings using detailed published data concentrating on the likely frequency of cross-contamination events.
Results: Our model predicts that as the TB prevalence in the suspect population increases there is an exponential increase in the risk of MTB cross-contamination events expected in otherwise negative samples, even with equivalent technical performance of the laboratories. Quality control and strict cross-contamination measures become increasingly critical as the burden of MTB infection among TB suspects increases. Even under optimal conditions the realistically achievable specificity of these systems in high burden settings will likely be significantly below that obtained in low TB burden laboratories.
Conclusions: Liquid culture systems can play a valuable role in TB case detection in laboratories in high burden settings, but laboratory workers, policy makers and clinicians should be aware of the increased risks, independent of laboratory proficiency, of cross-contamination events in high burden settings.
Figures
References
-
- Anthony RM, Cobelens FG, Gebhard A, Klatser PR, Lumb R, Rüsch-Gerdes S, van Soolingen D. Liquid culture for Mycobacterium tuberculosis: proceed but with caution. Int J Tuberc Lung Dis. 2009;13:1051–1053. - PubMed
-
- Urbanczik R, Rieder HL. Scaling up tuberculosis culture services: a precautionary note. Int J Tuberc Lung Dis. 2009;13:799–800. - PubMed
-
- Martinez M, Garcia de Viedma D, Alonso M, Adrés S, Bouza E, Cabezas T, Cabeza I, Reyes A, Sánchez-Yebra W, Rodríguez M, Sánchez MI, Rogado MC, Fernández R, Peñafiel T, Martínez J, Barroso P, Lucerna MA, Diez LF, Gutiérrez C. Impact of laboratory cross-contamination on molecular epidemiology studies of tuberculosis. J Clin Microbiol. 2006;44:2967–2969. doi: 10.1128/JCM.00754-06. - DOI - PMC - PubMed
-
- De Boer AS, Blommerde B, de Haas PE, Sebek MM, Lambregts-van Weezenbeek KS, Dessens M, van Soolingen D. False-positive Mycobacterium tuberculosis cultures in 44 laboratories in the Netherlands (1993 to 2000): Incidence, risk factors, and consequences. J Clin Microbiol. 2002;40:4004–4009. doi: 10.1128/JCM.40.11.4004-4009.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

