Plasmodium falciparum spermidine synthase inhibition results in unique perturbation-specific effects observed on transcript, protein and metabolite levels
- PMID: 20385001
- PMCID: PMC2867828
- DOI: 10.1186/1471-2164-11-235
Plasmodium falciparum spermidine synthase inhibition results in unique perturbation-specific effects observed on transcript, protein and metabolite levels
Abstract
Background: Plasmodium falciparum, the causative agent of severe human malaria, has evolved to become resistant to previously successful antimalarial chemotherapies, most notably chloroquine and the antifolates. The prevalence of resistant strains has necessitated the discovery and development of new chemical entities with novel modes-of-action. Although much effort has been invested in the creation of analogues based on existing drugs and the screening of chemical and natural compound libraries, a crucial shortcoming in current Plasmodial drug discovery efforts remains the lack of an extensive set of novel, validated drug targets. A requirement of these targets (or the pathways in which they function) is that they prove essential for parasite survival. The polyamine biosynthetic pathway, responsible for the metabolism of highly abundant amines crucial for parasite growth, proliferation and differentiation, is currently under investigation as an antimalarial target. Chemotherapeutic strategies targeting this pathway have been successfully utilized for the treatment of Trypanosomes causing West African sleeping sickness. In order to further evaluate polyamine depletion as possible antimalarial intervention, the consequences of inhibiting P. falciparum spermidine synthase (PfSpdSyn) were examined on a morphological, transcriptomic, proteomic and metabolic level.
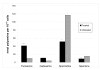
Results: Morphological analysis of P. falciparum 3D7 following application of the PfSpdSyn inhibitor cyclohexylamine confirmed that parasite development was completely arrested at the early trophozoite stage. This is in contrast to untreated parasites which progressed to late trophozoites at comparable time points. Global gene expression analyses confirmed a transcriptional arrest in the parasite. Several of the differentially expressed genes mapped to the polyamine biosynthetic and associated metabolic pathways. Differential expression of corresponding parasite proteins involved in polyamine biosynthesis was also observed. Most notably, uridine phosphorylase, adenosine deaminase, lysine decarboxylase (LDC) and S-adenosylmethionine synthetase were differentially expressed at the transcript and/or protein level. Several genes in associated metabolic pathways (purine metabolism and various methyltransferases) were also affected. The specific nature of the perturbation was additionally reflected by changes in polyamine metabolite levels.
Conclusions: This study details the malaria parasite's response to PfSpdSyn inhibition on the transcriptomic, proteomic and metabolic levels. The results corroborate and significantly expand previous functional genomics studies relating to polyamine depletion in this parasite. Moreover, they confirm the role of transcriptional regulation in P. falciparum, particularly in this pathway. The findings promote this essential pathway as a target for antimalarial chemotherapeutic intervention strategies.
Figures


Similar articles
-
Assessing the polyamine metabolism of Plasmodium falciparum as chemotherapeutic target.Mol Biochem Parasitol. 2008 Jul;160(1):1-7. doi: 10.1016/j.molbiopara.2008.03.008. Epub 2008 Mar 25. Mol Biochem Parasitol. 2008. PMID: 18455248 Review.
-
Functional consequences of perturbing polyamine metabolism in the malaria parasite, Plasmodium falciparum.Amino Acids. 2010 Feb;38(2):633-44. doi: 10.1007/s00726-009-0424-7. Epub 2009 Dec 9. Amino Acids. 2010. PMID: 19997948 Review.
-
A novel inhibitor of Plasmodium falciparum spermidine synthase: a twist in the tail.Malar J. 2015 Feb 5;14:54. doi: 10.1186/s12936-015-0572-z. Malar J. 2015. PMID: 25651815 Free PMC article.
-
The spermidine synthase of the malaria parasite Plasmodium falciparum: molecular and biochemical characterisation of the polyamine synthesis enzyme.Mol Biochem Parasitol. 2005 Aug;142(2):224-36. doi: 10.1016/j.molbiopara.2005.04.004. Mol Biochem Parasitol. 2005. PMID: 15913804
-
Screening of potential targets in Plasmodium falciparum using stage-specific metabolic network analysis.Mol Divers. 2015 Nov;19(4):991-1002. doi: 10.1007/s11030-015-9632-0. Epub 2015 Aug 25. Mol Divers. 2015. PMID: 26303382
Cited by
-
In vitro anti-plasmodial activity of Dicoma anomala subsp. gerrardii (Asteraceae): identification of its main active constituent, structure-activity relationship studies and gene expression profiling.Malar J. 2011 Oct 11;10:295. doi: 10.1186/1475-2875-10-295. Malar J. 2011. PMID: 21985233 Free PMC article.
-
A Francisella tularensis locus required for spermine responsiveness is necessary for virulence.Infect Immun. 2011 Sep;79(9):3665-76. doi: 10.1128/IAI.00135-11. Epub 2011 Jun 13. Infect Immun. 2011. PMID: 21670171 Free PMC article.
-
An Undefined Interaction between Polyamines and Heat Shock Proteins Leads to Cellular Protection in Plasmodium falciparum and Proliferating Cells in Various Organisms.Molecules. 2023 Feb 10;28(4):1686. doi: 10.3390/molecules28041686. Molecules. 2023. PMID: 36838674 Free PMC article. Review.
-
Putrescine-dependent re-localization of TvCP39, a cysteine proteinase involved in Trichomonas vaginalis cytotoxicity.PLoS One. 2014 Sep 24;9(9):e107293. doi: 10.1371/journal.pone.0107293. eCollection 2014. PLoS One. 2014. PMID: 25251406 Free PMC article.
-
Metabolomics and malaria biology.Mol Biochem Parasitol. 2011 Feb;175(2):104-11. doi: 10.1016/j.molbiopara.2010.09.008. Epub 2010 Oct 21. Mol Biochem Parasitol. 2011. PMID: 20970461 Free PMC article. Review.
References
-
- Takatsuka Y, Yamaguchi Y, M O, Kamio Y. Gene cloning and molecular characterization of lysine decarboxylase from Selenomonas ruminantium delineate its evolutionary relationship to ornithine decarboxylases from eukaryotes. J Bacteriol. 2000;182(23):6732–6741. doi: 10.1128/JB.182.23.6732-6741.2000. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

