Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo
- PMID: 20385088
- PMCID: PMC3641559
- DOI: 10.1016/j.molcel.2010.01.042
Highly compacted chromatin formed in vitro reflects the dynamics of transcription activation in vivo
Abstract
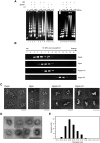
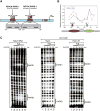
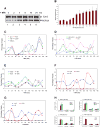
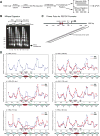
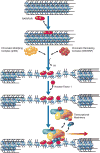
High-order chromatin was reconstituted in vitro. This species reflects the criteria associated with transcriptional regulation in vivo. Histone H1 was determinant to formation of condensed structures, with deacetylated histones giving rise to highly compacted chromatin that approximated 30 nm fibers as evidenced by electron microscopy. Using the PEPCK promoter, we validated the integrity of these templates that were refractory to transcription by attaining transcription through the progressive action of the pertinent factors. The retinoic acid receptor binds to highly compacted chromatin, but the NF1 transcription factor binds only after histone acetylation by p300 and SWI/SNF-mediated nucleosome mobilization, reflecting the in vivo case. Mapping studies revealed the same pattern of nucleosomal repositioning on the PEPCK promoter in vitro and in vivo, correlating with NF1 binding and transcription. The reconstitution of such highly compacted "30 nm" chromatin that mimics in vivo characteristics should advance studies of its conversion to a transcriptionally active form.
2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Archer TK, Lefebvre P, Wolford RG, Hager GL. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992;255:1573–1576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

