Up-regulation of striatal adenosine A(2A) receptors with iron deficiency in rats: effects on locomotion and cortico-striatal neurotransmission
- PMID: 20385128
- PMCID: PMC2885550
- DOI: 10.1016/j.expneurol.2010.04.004
Up-regulation of striatal adenosine A(2A) receptors with iron deficiency in rats: effects on locomotion and cortico-striatal neurotransmission
Abstract
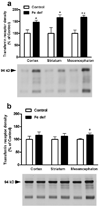
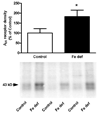
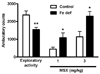
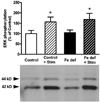
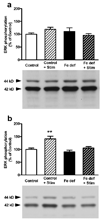
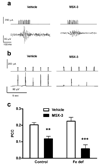
Brain iron deficiency leads to altered dopaminergic function in experimental animals, which can provide a mechanistic explanation for iron deficiency-related human sensory-motor disorders, such as Restless Legs Syndrome (RLS). However, mechanisms linking both conditions have not been determined. Considering the strong modulation exerted by adenosine on dopamine signaling, one connection could involve changes in adenosine receptor expression or function. In the striatum, presynaptic A(2A) receptors are localized in glutamatergic terminals contacting GABAergic dynorphinergic neurons and their function can be analyzed by the ability of A(2A) receptor antagonists to block the motor output induced by cortical electrical stimulation. Postsynaptic A(2A) receptors are localized in the dendritic field of GABAergic enkephalinergic neurons and their function can be analyzed by studying the ability of A(2A) receptor antagonists to produce locomotor activity and to counteract striatal ERK1/2 phosphorylation induced by cortical electrical stimulation. Increased density of striatal A(2A) receptors was found in rats fed during 3 weeks with an iron-deficient diet during the post-weaning period. In iron-deficient rats, the selective A(2A) receptor antagonist MSX-3, at doses of 1 and 3 mg/kg, was more effective at blocking motor output induced by cortical electrical stimulation (presynaptic A(2A) receptor-mediated effect) and at enhancing locomotor activation and blocking striatal ERK phosphorylation induced by cortical electrical stimulation (postsynaptic A(2A) receptor-mediated effects). These results indicate that brain iron deficiency induces a functional up-regulation of both striatal pre- and postsynaptic A(2A) receptor, which could be involved in sensory-motor disorders associated with iron deficiency such as RLS.
Copyright 2010. Published by Elsevier Inc.
Figures
Similar articles
-
Functional changes in postsynaptic adenosine A(2A) receptors during early stages of a rat model of Huntington disease.Exp Neurol. 2011 Nov;232(1):76-80. doi: 10.1016/j.expneurol.2011.08.005. Epub 2011 Aug 16. Exp Neurol. 2011. PMID: 21867705 Free PMC article.
-
Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum.Neuropharmacology. 2011 Oct-Nov;61(5-6):967-74. doi: 10.1016/j.neuropharm.2011.06.025. Epub 2011 Jul 5. Neuropharmacology. 2011. PMID: 21752341 Free PMC article.
-
Adenosine receptors as markers of brain iron deficiency: Implications for Restless Legs Syndrome.Neuropharmacology. 2016 Dec;111:160-168. doi: 10.1016/j.neuropharm.2016.09.002. Epub 2016 Sep 4. Neuropharmacology. 2016. PMID: 27600688 Free PMC article.
-
Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function.Neurology. 2003 Dec 9;61(11 Suppl 6):S12-8. doi: 10.1212/01.wnl.0000095205.33940.99. Neurology. 2003. PMID: 14663003 Review.
-
Adenosine-cannabinoid receptor interactions. Implications for striatal function.Br J Pharmacol. 2010 Jun;160(3):443-53. doi: 10.1111/j.1476-5381.2010.00723.x. Br J Pharmacol. 2010. PMID: 20590556 Free PMC article. Review.
Cited by
-
Differential effects of presynaptic versus postsynaptic adenosine A2A receptor blockade on Δ9-tetrahydrocannabinol (THC) self-administration in squirrel monkeys.J Neurosci. 2014 May 7;34(19):6480-4. doi: 10.1523/JNEUROSCI.5073-13.2014. J Neurosci. 2014. PMID: 24806674 Free PMC article.
-
Striatal pre- and postsynaptic profile of adenosine A(2A) receptor antagonists.PLoS One. 2011 Jan 11;6(1):e16088. doi: 10.1371/journal.pone.0016088. PLoS One. 2011. PMID: 21264319 Free PMC article.
-
Brain Iron Deficiency Changes the Stoichiometry of Adenosine Receptor Subtypes in Cortico-Striatal Terminals: Implications for Restless Legs Syndrome.Molecules. 2022 Feb 23;27(5):1489. doi: 10.3390/molecules27051489. Molecules. 2022. PMID: 35268590 Free PMC article.
-
Adenosine mechanisms and hypersensitive corticostriatal terminals in restless legs syndrome. Rationale for the use of inhibitors of adenosine transport.Adv Pharmacol. 2019;84:3-19. doi: 10.1016/bs.apha.2018.12.005. Epub 2019 Jan 18. Adv Pharmacol. 2019. PMID: 31229176 Free PMC article.
-
Advances in CNS PET: the state-of-the-art for new imaging targets for pathophysiology and drug development.Eur J Nucl Med Mol Imaging. 2020 Feb;47(2):451-489. doi: 10.1007/s00259-019-04488-0. Epub 2019 Sep 21. Eur J Nucl Med Mol Imaging. 2020. PMID: 31541283 Free PMC article. Review.
References
-
- Allen RP, Walter AS, Montplaisir J, Hening W, Myers A, Bell TJ, Ferini-Strambi L. Restless legs syndrome prevalence and impact: REST general population study. Arch. Intern. Med. 2005;165:1286–1292. - PubMed
-
- Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. - PubMed
-
- Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–391. - PubMed
-
- Angulo-Kinzler RM, Peirano P, Lin E, Algarin C, Garrido M, Lozoff B. Twenty-four-hour motor activity in human infants with and without iron deficiency anemia. Early Hum. Dev. 2002;70:85–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

