Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties
- PMID: 20385260
- PMCID: PMC2910244
- DOI: 10.1016/j.actbio.2010.03.040
Influence of cell-adhesive peptide ligands on poly(ethylene glycol) hydrogel physical, mechanical and transport properties
Abstract
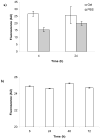
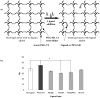
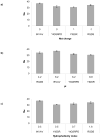
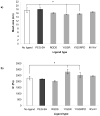
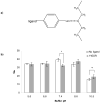
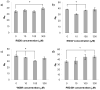
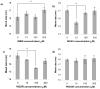
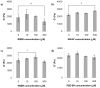
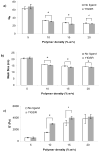
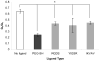
Synthetic three-dimensional scaffolds for cell and tissue engineering routinely utilize peptide ligands to provide sites for cell adhesion and to promote cellular activity. Given the fact that recent studies have dedicated great attention to the mechanisms by which cell behavior is influenced by various ligands and scaffold material properties, it is surprising that little work to date has been carried out to investigate the influence of covalently bound ligands on hydrogel material properties. Herein we report the influence of three common ligands utilized in tissue engineering, namely RGD, YIGSR and IKVAV, on the mechanical properties of cross-linked poly(ethylene glycol) (PEG) hydrogels. The effect of the ligands on hydrogel storage modulus, swelling ratio, mesh size and also on the diffusivity of bovine serum albumin through the hydrogel were investigated in detail. We identified conditions under which these ligands strikingly influence the properties of the material. The extent of influence and whether the ligand increases or decreases a specific property is linked to ligand type and concentration. Further, we pinpoint mechanisms by which the ligands interact with the PEG network. This work thus provides specific evidence for interactions between peptide ligands and cross-linked PEG hydrogels that have a significant impact on hydrogel material and transport properties. As a result, this work may have important implications for interpreting cell experiments carried out with ligand-modified hydrogels, because the addition of ligand may affect not only the scaffold's biological properties, but also key physical properties of the system.
2010 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Influence of FGF2 and PEG hydrogel matrix properties on hMSC viability and spreading.J Biomed Mater Res A. 2010 Jun 1;93(3):1110-23. doi: 10.1002/jbm.a.32601. J Biomed Mater Res A. 2010. PMID: 19768790 Free PMC article.
-
Biomimetic-engineered poly (ethylene glycol) hydrogel for smooth muscle cell migration.Tissue Eng Part A. 2014 Feb;20(3-4):864-73. doi: 10.1089/ten.TEA.2013.0050. Epub 2014 Jan 9. Tissue Eng Part A. 2014. PMID: 24093717 Free PMC article.
-
Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering.Acta Biomater. 2015 Mar;14:11-21. doi: 10.1016/j.actbio.2014.11.042. Epub 2014 Nov 26. Acta Biomater. 2015. PMID: 25433168 Free PMC article.
-
Design properties of hydrogel tissue-engineering scaffolds.Expert Rev Med Devices. 2011 Sep;8(5):607-26. doi: 10.1586/erd.11.27. Expert Rev Med Devices. 2011. PMID: 22026626 Free PMC article. Review.
-
Mechanical properties of cellularly responsive hydrogels and their experimental determination.Adv Mater. 2010 Aug 17;22(31):3484-94. doi: 10.1002/adma.200904179. Adv Mater. 2010. PMID: 20473984 Free PMC article. Review.
Cited by
-
Photoinitiator-free synthesis of endothelial cell-adhesive and enzymatically degradable hydrogels.Acta Biomater. 2015 Feb;13:52-60. doi: 10.1016/j.actbio.2014.11.012. Epub 2014 Nov 13. Acta Biomater. 2015. PMID: 25462848 Free PMC article.
-
Maximizing phenotype constraint and extracellular matrix production in primary human chondrocytes using arginine-glycine-aspartate concentration gradient hydrogels.Acta Biomater. 2013 Jul;9(7):7420-8. doi: 10.1016/j.actbio.2013.04.005. Epub 2013 Apr 6. Acta Biomater. 2013. PMID: 23567942 Free PMC article.
-
In Vitro Screening of Molecularly Engineered Polyethylene Glycol Hydrogels for Cartilage Tissue Engineering using Periosteum-Derived and ATDC5 Cells.Int J Mol Sci. 2018 Oct 26;19(11):3341. doi: 10.3390/ijms19113341. Int J Mol Sci. 2018. PMID: 30373138 Free PMC article.
-
Slow hydrogel matrix degradation enhances salivary gland mimetic phenotype.Acta Biomater. 2023 Aug;166:187-200. doi: 10.1016/j.actbio.2023.05.005. Epub 2023 May 5. Acta Biomater. 2023. PMID: 37150277 Free PMC article.
-
Design of Injectable Materials to Improve Stem Cell Transplantation.Curr Stem Cell Rep. 2016 Sep;2(3):207-220. doi: 10.1007/s40778-016-0058-0. Epub 2016 Jul 1. Curr Stem Cell Rep. 2016. PMID: 28868235 Free PMC article.
References
-
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–3. - PubMed
-
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–7. - PubMed
-
- Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

