Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts
- PMID: 20385637
- PMCID: PMC2884182
- DOI: 10.1542/peds.2009-2317
Comparative safety of antidepressant agents for children and adolescents regarding suicidal acts
Abstract
Objective: The objective of this study was to assess the risk of suicide attempts and suicides after initiation of antidepressant medication use by children and adolescents, for individual agents.
Methods: We conducted a 9-year cohort study by using population-wide data from British Columbia. We identified new users of antidepressants who were 10 to 18 years of age with a recorded diagnosis of depression. Study outcomes were hospitalization attributable to intentional self-harm and suicide death.
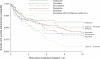
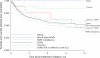
Results: Of 20,906 children who initiated antidepressant therapy, 16,774 (80%) had no previous antidepressant use. During the first year of use, we observed 266 attempted and 3 completed suicides, which yielded an event rate of 27.04 suicidal acts per 1000 person-years (95% confidence interval [CI]: 23.9-30.5 suicidal acts per 1000 person-years). There were no meaningful differences in the rate ratios (RRs) comparing fluoxetine with citalopram (RR: 0.97 [95% CI: 0.54-1.76]), fluvoxamine (RR: 1.05 [95% CI: 0.46-2.43]), paroxetine (RR: 0.80 [95% CI: 0.47-1.37]), and sertraline (RR: 1.02 [95% CI: 0.56-1.84]). Tricyclic agents showed risks similar to those of selective serotonin reuptake inhibitors (RR: 0.92 [95% CI: 0.43-2.00]).
Conclusion: Our finding of equal event rates among antidepressant agents supports the decision of the Food and Drug Administration to include all antidepressants in the black box warning regarding potentially increased suicidality risk for children and adolescents beginning use of antidepressants.
Figures
Comment in
-
Expose, heed, and coordinate care: priorities for mental health promotion and suicide prevention.Pediatrics. 2010 May;125(5):1064-5. doi: 10.1542/peds.2010-0610. Epub 2010 Apr 12. Pediatrics. 2010. PMID: 20385638 No abstract available.
References
-
- Food and Drug Administration Summary minutes of the September 13–14, 2004 Center for Drug Evaluation and Research Pharmachopharmacologic Drugs Advisory Committee and the FDA Pediatric Advisory Committee. [February 1, 2005]. Available at: www.fda.gov/ohrms/dockets/ac/04/minutes/2004-4065M1_Final.htm.
-
- Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with anti-depressant drugs. Arch Gen Psychiatry. 2006;63(3):332–339. - PubMed
-
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Tanskanen A, Haukka J. Antidepressants and the risk of suicide, attempted suicide, and overall mortality in a nationwide cohort. Arch Gen Psychiatry. 2006;63(12):1358–1367. - PubMed
-
- Olfson M, Marcus SC, Shaffer D. Antidepressant drug therapy and suicide in severely depressed children and adults: a case-control study. Arch Gen Psychiatry. 2006;63(8):865–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

