Rickettsia rickettsii infection of human macrovascular and microvascular endothelial cells reveals activation of both common and cell type-specific host response mechanisms
- PMID: 20385756
- PMCID: PMC2876542
- DOI: 10.1128/IAI.01335-09
Rickettsia rickettsii infection of human macrovascular and microvascular endothelial cells reveals activation of both common and cell type-specific host response mechanisms
Abstract
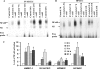
Although inflammation and altered barrier functions of the vasculature, due predominantly to the infection of endothelial cell lining of small and medium-sized blood vessels, represent salient pathological features of human rickettsioses, the interactions between pathogenic rickettsiae and microvascular endothelial cells remain poorly understood. We have investigated the activation of nuclear transcription factor-kappa B (NF-kappaB) and p38 mitogen-activated protein (MAP) kinase, expression of heme oxygenase 1 (HO-1) and cyclooxygenase 2 (COX-2), and secretion of chemokines and prostaglandins after Rickettsia rickettsii infection of human cerebral, dermal, and pulmonary microvascular endothelial cells in comparison with pulmonary artery cells of macrovascular origin. NF-kappaB and p38 kinase activation and increased HO-1 mRNA expression were clearly evident in all cell types, along with relatively similar susceptibility to R. rickettsii infection in vitro but considerable variations in the intensities/kinetics of the aforementioned host responses. As expected, the overall activation profiles of macrovascular endothelial cells derived from human pulmonary artery and umbilical vein were nearly identical. Interestingly, cerebral endothelial cells displayed a marked refractoriness in chemokine production and secretion, while all other cell types secreted various levels of interleukin-8 (IL-8) and monocyte chemoattractant protein 1 (MCP-1) in response to infection. A unique feature of all microvascular endothelial cells was the lack of induced COX-2 expression and resultant inability to secrete prostaglandin E(2) after R. rickettsii infection. Comparative evaluation thus yields the first experimental evidence for the activation of both common and unique cell type-specific host response mechanisms in macrovascular and microvascular endothelial cells infected with R. rickettsii, a prototypical species known to cause Rocky Mountain spotted fever in humans.
Figures
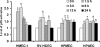

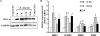

Similar articles
-
Infection of human endothelial cells with spotted Fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins.Infect Immun. 2006 Sep;74(9):5067-74. doi: 10.1128/IAI.00182-06. Infect Immun. 2006. PMID: 16926398 Free PMC article.
-
Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: regulation by nuclear transcription factor NF-kappaB.Int J Med Microbiol. 2005 Aug;295(4):267-78. doi: 10.1016/j.ijmm.2005.05.006. Int J Med Microbiol. 2005. PMID: 16128401
-
NF-kappaB activation during Rickettsia rickettsii infection of endothelial cells involves the activation of catalytic IkappaB kinases IKKalpha and IKKbeta and phosphorylation-proteolysis of the inhibitor protein IkappaBalpha.Infect Immun. 2005 Jan;73(1):155-65. doi: 10.1128/IAI.73.1.155-165.2005. Infect Immun. 2005. PMID: 15618150 Free PMC article.
-
Similarities and differences in host cell signaling following infection with different Rickettsia species.Ann N Y Acad Sci. 2005 Dec;1063:203-6. doi: 10.1196/annals.1355.030. Ann N Y Acad Sci. 2005. PMID: 16481515 Review.
-
Host-cell interactions with pathogenic Rickettsia species.Future Microbiol. 2009 Apr;4(3):323-39. doi: 10.2217/fmb.09.6. Future Microbiol. 2009. PMID: 19327117 Free PMC article. Review.
Cited by
-
Global Transcriptomic Profiling of Pulmonary Gene Expression in an Experimental Murine Model of Rickettsia conorii Infection.Genes (Basel). 2019 Mar 8;10(3):204. doi: 10.3390/genes10030204. Genes (Basel). 2019. PMID: 30857242 Free PMC article.
-
In silico construction of a multi-epitope vaccine (RGME-VAC/ATS-1) against the Rickettsia genus using immunoinformatics.Mem Inst Oswaldo Cruz. 2025 Mar 21;120:e240201. doi: 10.1590/0074-02760240201. eCollection 2025. Mem Inst Oswaldo Cruz. 2025. PMID: 40136144 Free PMC article.
-
MicroRNA-424 regulates the expression of CX3CL1 (fractalkine) in human microvascular endothelial cells during Rickettsia rickettsii infection.Biochem Biophys Rep. 2021 Jan 6;25:100897. doi: 10.1016/j.bbrep.2020.100897. eCollection 2021 Mar. Biochem Biophys Rep. 2021. PMID: 33490646 Free PMC article.
-
Identification and Characterization of Novel Small RNAs in Rickettsia prowazekii.Front Microbiol. 2016 Jun 8;7:859. doi: 10.3389/fmicb.2016.00859. eCollection 2016. Front Microbiol. 2016. PMID: 27375581 Free PMC article.
-
Heme binding proteins of Bartonella henselae are required when undergoing oxidative stress during cell and flea invasion.PLoS One. 2012;7(10):e48408. doi: 10.1371/journal.pone.0048408. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144761 Free PMC article.
References
-
- Ades, E. W., F. J. Candal, R. A. Swerlick, V. G. George, S. Summers, D. C. Bosse, and T. J. Lawley. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J. Investig. Dermatol. 99:683-690. - PubMed
-
- Aird, W. C. 2004. Endothelial cell heterogeneity: a case for nature and nurture. Blood 103:3994-3995.
-
- Aird, W. C. 2008. Endothelium in health and disease. Pharmacol. Rep. 60:139-143. - PubMed
-
- Brigham, K. L. 1990. Oxidant stress and adult respiratory distress syndrome. Eur. Respir. J. 11:S482-S484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

