Role of lipid A acylation in Yersinia enterocolitica virulence
- PMID: 20385763
- PMCID: PMC2876571
- DOI: 10.1128/IAI.01417-09
Role of lipid A acylation in Yersinia enterocolitica virulence
Abstract
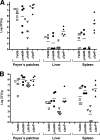
Yersinia enterocolitica is an important human pathogen. Y. enterocolitica must adapt to the host environment, and temperature is an important cue regulating the expression of most Yersinia virulence factors. Here, we report that Y. enterocolitica 8081 serotype O:8 synthesized tetra-acylated lipid A at 37 degrees C but that hexa-acylated lipid A predominated at 21 degrees C. By mass spectrometry and genetic methods, we have shown that the Y. enterocolitica msbB, htrB, and lpxP homologues encode the acyltransferases responsible for the addition of C(12), C(14) and C(16:1), respectively, to lipid A. The expression levels of the acyltransferases were temperature regulated. Levels of expression of msbB and lpxP were higher at 21 degrees C than at 37 degrees C, whereas the level of expression of htrB was higher at 37 degrees C. At 21 degrees C, an lpxP mutant was the strain most susceptible to polymyxin B, whereas at 37 degrees C, an htrB mutant was the most susceptible. We present evidence that the lipid A acylation status affects the expression of Yersinia virulence factors. Thus, expression of flhDC, the flagellar master regulatory operon, was downregulated in msbB and lpxP mutants, with a concomitant decrease in motility. Expression of the phospholipase yplA was also downregulated in both mutants. inv expression was downregulated in msbB and htrB mutants, and consistent with this finding, invasion of HeLa cells was diminished. However, the expression of rovA, the positive regulator of inv, was not affected in the mutants. The levels of pYV-encoded virulence factors Yops and YadA in the acyltransferase mutants were not affected. Finally, we show that only the htrB mutant was attenuated in vivo.
Figures




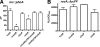
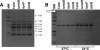
Similar articles
-
Deciphering the acylation pattern of Yersinia enterocolitica lipid A.PLoS Pathog. 2012;8(10):e1002978. doi: 10.1371/journal.ppat.1002978. Epub 2012 Oct 25. PLoS Pathog. 2012. PMID: 23133372 Free PMC article.
-
Lipopolysaccharide O antigen status of Yersinia enterocolitica O:8 is essential for virulence and absence of O antigen affects the expression of other Yersinia virulence factors.Mol Microbiol. 2004 Apr;52(2):451-69. doi: 10.1111/j.1365-2958.2004.03987.x. Mol Microbiol. 2004. PMID: 15066033
-
Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation.J Bacteriol. 2006 Feb;188(4):1381-8. doi: 10.1128/JB.188.4.1381-1388.2006. J Bacteriol. 2006. PMID: 16452420 Free PMC article.
-
Interactions between Yersinia enterocolitica and the host with special reference to virulence plasmid encoded adhesion and humoral immunity.Dan Med Bull. 1992 Apr;39(2):155-72. Dan Med Bull. 1992. PMID: 1611921 Review.
-
Yersinia enterocolitica, a primary model for bacterial invasiveness.Rev Infect Dis. 1987 Jan-Feb;9(1):64-87. doi: 10.1093/clinids/9.1.64. Rev Infect Dis. 1987. PMID: 3547579 Review.
Cited by
-
The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition.PLoS Pathog. 2012;8(5):e1002675. doi: 10.1371/journal.ppat.1002675. Epub 2012 May 10. PLoS Pathog. 2012. PMID: 22589715 Free PMC article.
-
Variation, Modification and Engineering of Lipid A in Endotoxin of Gram-Negative Bacteria.Int J Mol Sci. 2021 Feb 25;22(5):2281. doi: 10.3390/ijms22052281. Int J Mol Sci. 2021. PMID: 33668925 Free PMC article. Review.
-
RNA-Seq-based analysis of the physiologic cold shock-induced changes in Moraxella catarrhalis gene expression.PLoS One. 2013 Jul 2;8(7):e68298. doi: 10.1371/journal.pone.0068298. Print 2013. PLoS One. 2013. PMID: 23844181 Free PMC article.
-
Molecular Mechanisms of Bacterial Resistance to Antimicrobial Peptides in the Modern Era: An Updated Review.Microorganisms. 2024 Jun 21;12(7):1259. doi: 10.3390/microorganisms12071259. Microorganisms. 2024. PMID: 39065030 Free PMC article. Review.
-
Plague vaccines: new developments in an ongoing search.Appl Microbiol Biotechnol. 2021 Jun;105(12):4931-4941. doi: 10.1007/s00253-021-11389-6. Epub 2021 Jun 18. Appl Microbiol Biotechnol. 2021. PMID: 34142207 Free PMC article. Review.
References
-
- Alexeyev, M. F. 1999. The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26:824-826, 828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

