Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras
- PMID: 20385769
- PMCID: PMC2876686
- DOI: 10.1128/MCB.01652-09
Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras
Abstract
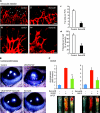
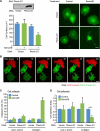
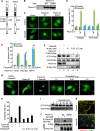
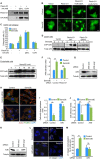
Recent studies revealed that a class III semaphorin, semaphorin 3E (Sema3E), acts through a single-pass transmembrane receptor, plexin D1, to provide a repulsive cue for plexin D1-expressing endothelial cells, thus providing a highly conserved and developmentally regulated signaling system guiding the growth of blood vessels. We show here that Sema3E acts as a potent inhibitor of adult and tumor-induced angiogenesis. Activation of plexin D1 by Sema3E causes the rapid disassembly of integrin-mediated adhesive structures, thereby inhibiting endothelial cell adhesion to the extracellular matrix (ECM) and causing the retraction of filopodia in endothelial tip cells. Sema3E acts on plexin D1 to initiate a two-pronged mechanism involving R-Ras inactivation and Arf6 stimulation, which affect the status of activation of integrins and their intracellular trafficking, respectively. Ultimately, our present study provides a molecular framework for antiangiogenesis signaling, thus impinging on a myriad of pathological conditions that are characterized by aberrant increase in neovessel formation, including cancer.
Figures


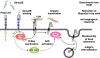
References
-
- Basile, J. R., K. Holmbeck, T. H. Bugge, and J. S. Gutkind. 2007. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J. Biol. Chem. 282:6899-6905. - PubMed
-
- Belperio, J. A., M. P. Keane, D. A. Arenberg, C. L. Addison, J. E. Ehlert, M. D. Burdick, and R. M. Strieter. 2000. CXC chemokines in angiogenesis. J. Leukoc. Biol. 68:1-8. - PubMed
-
- Cao, R., E. Brakenhielm, R. Pawliuk, D. Wariaro, M. J. Post, E. Wahlberg, P. Leboulch, and Y. Cao. 2003. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9:604-613. - PubMed
-
- Carmeliet, P. 2005. Angiogenesis in life, disease and medicine. Nature 438:932-936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
