The Cdk1 and Ime2 protein kinases trigger exit from meiotic prophase in Saccharomyces cerevisiae by inhibiting the Sum1 transcriptional repressor
- PMID: 20385771
- PMCID: PMC2876671
- DOI: 10.1128/MCB.01682-09
The Cdk1 and Ime2 protein kinases trigger exit from meiotic prophase in Saccharomyces cerevisiae by inhibiting the Sum1 transcriptional repressor
Abstract
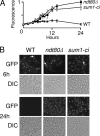
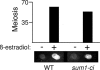
The induction of middle meiotic promoters is a key regulatory event in the life cycle of Saccharomyces cerevisiae that controls exit from prophase, meiosis, and spore formation. The Sum1 repressor and Ndt80 activator proteins control middle promoters by binding to overlapping DNA elements. NDT80 is controlled by a tightly regulated middle meiotic promoter through a positive autoregulatory loop and is repressed in vegetative cells by Sum1. It has previously been shown that the meiosis-specific kinase Ime2 promotes the removal of Sum1 from DNA. Here, we show that Sum1 is also regulated by the cyclin-dependent kinase, Cdk1. While sum1 phosphosite mutants that are insensitive to Cdk1 or Ime2 complete meiosis and form spores, a mutant that is insensitive to both Ime2 and Cdk1 (sum1-ci) blocks meiotic development in prophase with an ndt80Delta-like phenotype. Ectopic expression of NDT80 or mutation of a Sum1-binding element in the NDT80 promoter bypasses the sum1-ci block. Hst1 is a NAD(+)-dependent histone deacetylase that is linked to Sum1 by the Rfm1 tethering factor. Deletion of HST1 or RFM1 also bypasses the sum1-ci block. These results demonstrate that Sum1 functions as a key meiotic brake through the NDT80 promoter and that Cdk1 and Ime2 trigger exit from meiotic prophase by inhibiting the Sum1 transcriptional repression complex.
Figures





Similar articles
-
The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2012 Mar;76(1):1-15. doi: 10.1128/MMBR.05010-11. Microbiol Mol Biol Rev. 2012. PMID: 22390969 Free PMC article. Review.
-
The Ime2 protein kinase enhances the disassociation of the Sum1 repressor from middle meiotic promoters.Mol Cell Biol. 2009 Aug;29(16):4352-62. doi: 10.1128/MCB.00305-09. Epub 2009 Jun 15. Mol Cell Biol. 2009. PMID: 19528232 Free PMC article.
-
Multisite phosphorylation of the Sum1 transcriptional repressor by S-phase kinases controls exit from meiotic prophase in yeast.Mol Cell Biol. 2014 Jun;34(12):2249-63. doi: 10.1128/MCB.01413-13. Epub 2014 Apr 7. Mol Cell Biol. 2014. PMID: 24710277 Free PMC article.
-
Cdc7-Dbf4 is a gene-specific regulator of meiotic transcription in yeast.Mol Cell Biol. 2012 Jan;32(2):541-57. doi: 10.1128/MCB.06032-11. Epub 2011 Nov 21. Mol Cell Biol. 2012. PMID: 22106412 Free PMC article.
-
The Ime2 protein kinase family in fungi: more duties than just meiosis.Mol Microbiol. 2011 Apr;80(1):1-13. doi: 10.1111/j.1365-2958.2011.07575.x. Epub 2011 Mar 1. Mol Microbiol. 2011. PMID: 21306447 Review.
Cited by
-
Activation of the Smk1 mitogen-activated protein kinase by developmentally regulated autophosphorylation.Mol Cell Biol. 2013 Feb;33(4):688-700. doi: 10.1128/MCB.00973-12. Epub 2012 Dec 3. Mol Cell Biol. 2013. PMID: 23207907 Free PMC article.
-
Autophosphorylation of the Smk1 MAPK is spatially and temporally regulated by Ssp2 during meiotic development in yeast.Mol Biol Cell. 2015 Oct 1;26(19):3546-55. doi: 10.1091/mbc.E15-05-0322. Epub 2015 Aug 5. Mol Biol Cell. 2015. PMID: 26246597 Free PMC article.
-
Positive feedback of NDT80 expression ensures irreversible meiotic commitment in budding yeast.PLoS Genet. 2014 Jun 5;10(6):e1004398. doi: 10.1371/journal.pgen.1004398. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24901499 Free PMC article.
-
Repression of Middle Sporulation Genes in Saccharomyces cerevisiae by the Sum1-Rfm1-Hst1 Complex Is Maintained by Set1 and H3K4 Methylation.G3 (Bethesda). 2017 Dec 4;7(12):3971-3982. doi: 10.1534/g3.117.300150. G3 (Bethesda). 2017. PMID: 29066473 Free PMC article.
-
The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2012 Mar;76(1):1-15. doi: 10.1128/MMBR.05010-11. Microbiol Mol Biol Rev. 2012. PMID: 22390969 Free PMC article. Review.
References
-
- Chu, S., J. DeRisi, M. Eisen, J. Mulholland, D. Botstein, P. O. Brown, and I. Herskowitz. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699-705. (Erratum, 282: 1421.) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
