CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms
- PMID: 20385788
- PMCID: PMC2892953
- DOI: 10.1182/blood-2009-05-223727
CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms
Abstract
Activating alleles of Janus kinase 2 (JAK2) such as JAK2(V617F) are central to the pathogenesis of myeloproliferative neoplasms (MPN), suggesting that small molecule inhibitors targeting JAK2 may be therapeutically useful. We have identified an aminopyrimidine derivative (CYT387), which inhibits JAK1, JAK2, and tyrosine kinase 2 (TYK2) at low nanomolar concentrations, with few additional targets. Between 0.5 and 1.5muM CYT387 caused growth suppression and apoptosis in JAK2-dependent hematopoietic cell lines, while nonhematopoietic cell lines were unaffected. In a murine MPN model, CYT387 normalized white cell counts, hematocrit, spleen size, and restored physiologic levels of inflammatory cytokines. Despite the hematologic responses and reduction of the JAK2(V617F) allele burden, JAK2(V617F) cells persisted and MPN recurred upon cessation of treatment, suggesting that JAK2 inhibitors may be unable to eliminate JAK2(V617F) cells, consistent with preliminary results from clinical trials of JAK2 inhibitors in myelofibrosis. While the clinical benefit of JAK2 inhibitors may be substantial, not the least due to reduction of inflammatory cytokines and symptomatic improvement, our data add to increasing evidence that kinase inhibitor monotherapy of malignant disease is not curative, suggesting a need for drug combinations to optimally target the malignant cells.
Figures

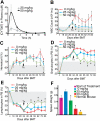



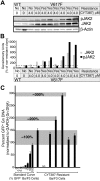
 ) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).
) and phospho JAK2 (■) were normalized to the levels observed in JAK2WT cells that are sensitive to CYT387. (C) Genomic DNA was isolated from CYT387-resistant Ba/F3 cells and levels of genomic GFP or GAPDH were assessed by quantitative PCR. A standard curve of varying ratios of GFP-positive/GFP-negative Ba/F3 cell mixtures was included as in Figure 5A. Levels of genomic GFP were normalized as in Figure 5 (normalized first to GAPDH, then to the highest value well on the standard curve) and are presented as percent GFP-positive. Values represent mean ± SEM (n = 3).Similar articles
-
Limited efficacy of BMS-911543 in a murine model of Janus kinase 2 V617F myeloproliferative neoplasm.Exp Hematol. 2015 Jul;43(7):537-45.e1-11. doi: 10.1016/j.exphem.2015.03.006. Epub 2015 Apr 24. Exp Hematol. 2015. PMID: 25912019 Free PMC article.
-
Efficacy of JAK1/2 inhibition in murine myeloproliferative neoplasms is not mediated by targeting oncogenic signaling.Nat Commun. 2025 May 24;16(1):4833. doi: 10.1038/s41467-025-60019-6. Nat Commun. 2025. PMID: 40413183 Free PMC article.
-
Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors.J Cell Mol Med. 2013 Nov;17(11):1397-409. doi: 10.1111/jcmm.12156. Epub 2013 Nov 19. J Cell Mol Med. 2013. PMID: 24251790 Free PMC article.
-
The role of Janus kinase 2 (JAK2) in myeloproliferative neoplasms: therapeutic implications.Leuk Res. 2013 Apr;37(4):465-72. doi: 10.1016/j.leukres.2012.12.006. Epub 2013 Jan 11. Leuk Res. 2013. PMID: 23313046 Review.
-
JAK inhibition in the myeloproliferative neoplasms: lessons learned from the bench and bedside.Hematology Am Soc Hematol Educ Program. 2013;2013:529-37. doi: 10.1182/asheducation-2013.1.529. Hematology Am Soc Hematol Educ Program. 2013. PMID: 24319228 Review.
Cited by
-
Small-molecule inhibitors of TBK1 serve as an adjuvant for a plasmid-launched live-attenuated yellow fever vaccine.Hum Vaccin Immunother. 2020 Sep 1;16(9):2196-2203. doi: 10.1080/21645515.2020.1765621. Epub 2020 Jun 23. Hum Vaccin Immunother. 2020. PMID: 32574095 Free PMC article.
-
Kinase inhibitors in the treatment of immune-mediated disease.F1000 Med Rep. 2012;4:5. doi: 10.3410/M4-5. Epub 2012 Mar 1. F1000 Med Rep. 2012. PMID: 22403586 Free PMC article.
-
Myeloproliferative neoplasm animal models.Hematol Oncol Clin North Am. 2012 Oct;26(5):1065-81. doi: 10.1016/j.hoc.2012.07.007. Epub 2012 Aug 21. Hematol Oncol Clin North Am. 2012. PMID: 23009938 Free PMC article. Review.
-
CHZ868, a Type II JAK2 Inhibitor, Reverses Type I JAK Inhibitor Persistence and Demonstrates Efficacy in Myeloproliferative Neoplasms.Cancer Cell. 2015 Jul 13;28(1):15-28. doi: 10.1016/j.ccell.2015.06.006. Cancer Cell. 2015. PMID: 26175413 Free PMC article.
-
JAK2 inhibition has different therapeutic effects according to myeloproliferative neoplasm development in mice.J Cell Mol Med. 2015 Nov;19(11):2564-74. doi: 10.1111/jcmm.12608. Epub 2015 Jul 14. J Cell Mol Med. 2015. PMID: 26176817 Free PMC article.
References
-
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
-
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790. - PubMed
-
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

