Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities
- PMID: 20385816
- PMCID: PMC2867891
- DOI: 10.1073/pnas.0912030107
Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities
Abstract
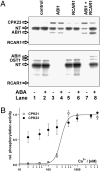
In response to drought stress, the phytohormone abscisic acid (ABA) induces stomatal closure. Thereby the stress hormone activates guard cell anion channels in a calcium-dependent, as well as -independent, manner. Open stomata 1 protein kinase (OST1) and ABI1 protein phosphatase (ABA insensitive 1) represent key components of calcium-independent ABA signaling. Recently, the guard cell anion channel SLAC1 was identified. When expressed heterologously SLAC1 remained electrically silent. Upon coexpression with Ca(2+)-independent OST1, however, SLAC1 anion channels appear activated in an ABI1-dependent manner. Mutants lacking distinct calcium-dependent protein kinases (CPKs) appeared impaired in ABA stimulation of guard cell ion channels, too. To study SLAC1 activation via the calcium-dependent ABA pathway, we studied the SLAC1 response to CPKs in the Xenopus laevis oocyte system. Split YFP-based protein-protein interaction assays, using SLAC1 as the bait, identified guard cell expressed CPK21 and 23 as major interacting partners. Upon coexpression of SLAC1 with CPK21 and 23, anion currents document SLAC1 stimulation by these guard cell protein kinases. Ca(2+)-sensitive activation of SLAC1, however, could be assigned to the CPK21 pathway only because CPK23 turned out to be rather Ca(2+)-insensitive. In line with activation by OST1, CPK activation of the guard cell anion channel was suppressed by ABI1. Thus the CPK and OST1 branch of ABA signal transduction in guard cells seem to converge on the level of SLAC1 under the control of the ABI1/ABA-receptor complex.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Roelfsema MR, Hedrich R. In the light of stomatal opening: New insights into 'the Watergate'. New Phytol. 2005;167:665–691. - PubMed
-
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waner D. Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. - PubMed
-
- Negi J, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:483–486. - PubMed
-
- Saji S, et al. Disruption of a gene encoding C4-dicarboxylate transporter-like protein increases ozone sensitivity through deregulation of the stomatal response in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:2–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

