Final visual acuity results in the early treatment for retinopathy of prematurity study
- PMID: 20385926
- PMCID: PMC4162423
- DOI: 10.1001/archophthalmol.2010.72
Final visual acuity results in the early treatment for retinopathy of prematurity study
Erratum in
- Arch Ophthalmol. 2012 Jun;130(6):719
Abstract
Objective: To compare visual acuity at 6 years of age in eyes that received early treatment for high-risk prethreshold retinopathy of prematurity (ROP) with conventionally managed eyes.
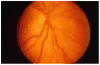
Methods: Infants with symmetrical, high-risk prethreshold ROP (n = 317) had one eye randomized to earlier treatment at high-risk prethreshold disease and the other eye managed conventionally, treated if ROP progressed to threshold severity. For asymmetric cases (n = 84), the high-risk prethreshold eye was randomized to either early treatment or conventional management. The main outcome measure was ETDRS visual acuity measured at 6 years of age by masked testers. Retinal structure was assessed as a secondary outcome.
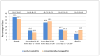
Results: Analysis of all subjects with high-risk prethreshold ROP showed no statistically significant benefit for early treatment (24.3% vs 28.6% [corrected] unfavorable outcome; P = .15). Analysis of 6-year visual acuity results according to the Type 1 and 2 clinical algorithm showed a benefit for Type 1 eyes (25.1% vs 32.8%; P = .02) treated early but not Type 2 eyes (23.6% vs 19.4%; P = .37). Early-treated eyes showed a significantly better structural outcome compared with conventionally managed eyes (8.9% vs 15.2% unfavorable outcome; P < .001), with no greater risk of ocular complications.
Conclusions: Early treatment for Type 1 high-risk prethreshold eyes improved visual acuity outcomes at 6 years of age. Early treatment for Type 2 high-risk prethreshold eyes did not. Application to Clinical Practice Type 1 eyes, not Type 2 eyes, should be treated early. These results are particularly important considering that 52% of Type 2 high-risk prethreshold eyes underwent regression of ROP without requiring treatment. Trial Registration clinicaltrials.gov Identifier: NCT00027222.
Figures

References
-
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity: preliminary results. Pediatrics. 1988 May;81(5):697–706. - PubMed
-
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988 Apr;106(4):471–479. - PubMed
-
- The Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984 Aug;102(8):1130–1134. - PubMed
-
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. One-year outcome--structure and function. Arch Ophthalmol. 1990 Oct;108(10):1408–1416. - PubMed
-
- Good WV, Hardy RJ. The multicenter study of Early Treatment for Retinopathy of Prematurity (ETROP) Ophthalmology. 2001 Jun;108(6):1013–1014. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

