Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals
- PMID: 20386073
- PMCID: PMC7733239
- DOI: 10.3851/IMP1514
Antiviral activity and tolerability of amdoxovir with zidovudine in a randomized double-blind placebo-controlled study in HIV-1-infected individuals
Abstract
Background: Amdoxovir acts synergistically with zidovudine in vitro and the combination prevents or delays the selection of thymidine analogue and K65R mutations. In silico studies have shown that a reduced dose of zidovudine (200 mg) results in decreased zidovudine-monophosphate levels, associated with toxicity, while maintaining zidovudine-triphosphate levels, which are associated with antiviral effects. Here, we aimed to assess the short-term tolerability and antiviral activity of amdoxovir in combination with reduced and standard doses of zidovudine.
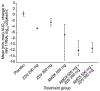
Methods: The study was a double-blind, placebo-controlled study in HIV-1-infected patients not receiving antiretroviral therapy and with plasma HIV-1 RNA > or =5,000 copies/ml. Patients were randomized to 10 days of twice-daily treatment with 200 mg zidovudine, 300 mg zidovudine, 500 mg amdoxovir, 500 mg amdoxovir plus 200 mg zidovudine or 500 mg amdoxovir plus 300 mg zidovudine. The mean change in viral load (VL) log(10) and area under the virus depletion curve (AUC(VL)) from baseline to day 10 were determined. Laboratory and clinical safety monitoring were performed.
Results: Twenty-four patients were enrolled. The mean VL log(10) change was 0.10 with placebo, -0.69 with zidovudine 200 mg, -0.55 with zidovudine 300 mg, -1.09 with amdoxovir, -2.00 with amdoxovir plus zidovudine (200 mg) and -1.69 with amdoxovir plus zidovudine (300 mg). Amdoxovir plus zidovudine (200 mg) was significantly more potent than amdoxovir monotherapy in AUC(VL) and mean VL decline (P=0.019 and P=0.021, respectively), suggesting synergy. There was markedly decreased VL variability with the combination compared with amdoxovir alone. All adverse events were mild to moderate.
Conclusion: The combination of amdoxovir plus zidovudine appeared synergistic with reduced VL variability. This combined therapy, including the use of a lower zidovudine dosage, warrants further development for the therapy of HIV infection.
Trial registration: ClinicalTrials.gov NCT00432016.
Conflict of interest statement
Disclosure statement
RFS, as an inventor of the amdoxovir technology and founder of RFS Pharma, has a financial interest that has been reviewed and approved by Emory University in compliance with its conflict of interest policies. RLM is a consultant to RFS Pharma. The remaining authors declare no competing interests.
Figures


References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services (Updated 3 November 2008; Accessed 12 December 2008). Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
-
- Pereira CF, Paridaen JT. Anti-HIV drug development – an overview. Curr Pharm Des 2004; 10:4005–4037. - PubMed
-
- Gu Z, Wainberg MA, Nguyen-Ba P, L’Heureux L, de Muys JM, Rando RF. Anti-HIV-1 activities of 1,3-dioxolane guanine and 2,6-diaminopurine dioxolane. Nucleosides Nucleotides 1999; 18:891–892. - PubMed
-
- Mewshaw JP, Myrick FT, Wakefield DACS, et al. Dioxolane guanosine, the active form of the prodrug diaminopurine dioxolane, is a potent inhibitor of drug-resistant HIV-1 isolates from patients for whom standard nucleoside therapy fails. J Acquir Immune Defic Syndr 2002; 29:11–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

