Epidemiology and pathophysiology of cancer-associated thrombosis
- PMID: 20386546
- PMCID: PMC3315367
- DOI: 10.1038/sj.bjc.6605599
Epidemiology and pathophysiology of cancer-associated thrombosis
Abstract
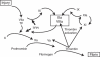
Venous thromboembolism (VTE) is a common complication in patients with malignant disease. First recognised by Bouillard in 1823 and later described by Trousseau in 1844, multiple studies have since provided considerable evidence for a clinical association between VTE and cancer. Across all cancers, the risk for VTE is elevated 7-fold; in certain malignancies, the risk for VTE may be increased up to 28-fold. Venous thromboembolism is the second leading cause of death in patients with cancer; among survivors, complications commonly include recurrent VTE and post-thrombotic syndrome, and (more rarely) chronic thromboembolic pulmonary hypertension, which are costly, and have a profound impact on the patient's quality of life. Tumour cells can activate blood coagulation through multiple mechanisms, including production of procoagulant, fibrinolytic, and proaggregating activities, release of proinflammatory and proangiogenic cytokines, and interacting directly with host vascular and blood cells (e.g., endothelial cells, leukocytes, and platelets) through adhesion molecules. Increasing evidence suggests that elements of the haemostatic system also have a direct role in eliciting or enhancing angiogenesis, cell survival, and metastasis. Despite the problem posed by VTE in the setting of cancer, it is evident that a significant number of oncologists do not recognise the link between cancer, its treatment, and thrombogenesis. On 22 May 2009, a group of UK-based physicians met in London, UK, to evaluate recent data on cancer thrombosis. This article (1 of 4) briefly reviews key data on the epidemiology and pathophysiology of VTE as a context for a discussion and consensus statement developed by meeting attendees, on the implications of this information for UK clinical practice.
Conflict of interest statement
KJ Pasi has received consulting fees from Bayer, Wyeth, Boehringer Ingelheim, Octapharma, and has also received lecture fees from Boehringer Ingelheim. S Noble has received grant support from Pfizer. All other related fees paid for consulting or lecture fees have been donated to charity.
Figures



Comment in
-
Determining the anti-coagulant-independent anti-cancer effects of heparin.Br J Cancer. 2010 Aug 10;103(4):593-4. doi: 10.1038/sj.bjc.6605808. Epub 2010 Jul 20. Br J Cancer. 2010. PMID: 20648011 Free PMC article. No abstract available.
References
-
- Barlogie B, Desikan R, Eddlemon P, Spencer T, Zeldis J, Munshi N, Badros A, Zangari M, Anaissie E, Epstein J, Shaughnessy J, Ayers D, Spoon D, Tricot G (2001) Extended survival in advanced and refractory multiple myeloma after single-agent thalidomide: identification of prognostic factors in a phase 2 study of 169 patients. Blood 98: 492–494 - PubMed
-
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR (2005) Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 293: 715–722 - PubMed
-
- Blom JW, Osanto S, Rosendaal FR (2004) The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2: 1760–1765 - PubMed
-
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, Trelle S, Weingart O, Bayliss S, Brunskill S, Djulbegovic B, Benett CL, Langensiepen S, Hyde C, Engert E (2006) Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 98: 708–714 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

