Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia
- PMID: 20386711
- PMCID: PMC2851657
- DOI: 10.1371/journal.ppat.1000835
Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia
Abstract
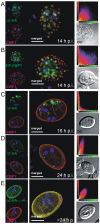
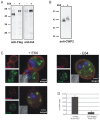
Controlled secretion of a protective extracellular matrix is required for transmission of the infective stage of a large number of protozoan and metazoan parasites. Differentiating trophozoites of the highly minimized protozoan parasite Giardia lamblia secrete the proteinaceous portion of the cyst wall material (CWM) consisting of three paralogous cyst wall proteins (CWP1-3) via organelles termed encystation-specific vesicles (ESVs). Phylogenetic and molecular data indicate that Diplomonads have lost a classical Golgi during reductive evolution. However, neogenesis of ESVs in encysting Giardia trophozoites transiently provides basic Golgi functions by accumulating presorted CWM exported from the ER for maturation. Based on this "minimal Golgi" hypothesis we predicted maturation of ESVs to a trans Golgi-like stage, which would manifest as a sorting event before regulated secretion of the CWM. Here we show that proteolytic processing of pro-CWP2 in maturing ESVs coincides with partitioning of CWM into two fractions, which are sorted and secreted sequentially with different kinetics. This novel sorting function leads to rapid assembly of a structurally defined outer cyst wall, followed by slow secretion of the remaining components. Using live cell microscopy we find direct evidence for condensed core formation in maturing ESVs. Core formation suggests that a mechanism controlled by phase transitions of the CWM from fluid to condensed and back likely drives CWM partitioning and makes sorting and sequential secretion possible. Blocking of CWP2 processing by a protease inhibitor leads to mis-sorting of a CWP2 reporter. Nevertheless, partitioning and sequential secretion of two portions of the CWM are unaffected in these cells. Although these cysts have a normal appearance they are not water resistant and therefore not infective. Our findings suggest that sequential assembly is a basic architectural principle of protective wall formation and requires minimal Golgi sorting functions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Rac Regulates Giardia lamblia Encystation by Coordinating Cyst Wall Protein Trafficking and Secretion.mBio. 2016 Aug 23;7(4):e01003-16. doi: 10.1128/mBio.01003-16. mBio. 2016. PMID: 27555307 Free PMC article.
-
Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia.J Biol Chem. 2006 Jun 30;281(26):18156-66. doi: 10.1074/jbc.M602081200. Epub 2006 Apr 12. J Biol Chem. 2006. PMID: 16611634
-
Neogenesis and maturation of transient Golgi-like cisternae in a simple eukaryote.J Cell Sci. 2009 Aug 15;122(Pt 16):2846-56. doi: 10.1242/jcs.049411. Epub 2009 Jul 21. J Cell Sci. 2009. PMID: 19622633
-
Protein trafficking in Giardia lamblia.Cell Microbiol. 2003 Jul;5(7):427-34. doi: 10.1046/j.1462-5822.2003.00284.x. Cell Microbiol. 2003. PMID: 12814433 Review.
-
[Secreted proteins of Giardia duodenalis--characteristic and role in biology of the parasite].Wiad Parazytol. 2005;51(1):15-9. Wiad Parazytol. 2005. PMID: 16841684 Review. Polish.
Cited by
-
Nuclear inheritance and genetic exchange without meiosis in the binucleate parasite Giardia intestinalis.J Cell Sci. 2012 May 15;125(Pt 10):2523-32. doi: 10.1242/jcs.103879. Epub 2012 Feb 24. J Cell Sci. 2012. PMID: 22366460 Free PMC article.
-
Staging Encystation Progression in Giardia lamblia Using Encystation-Specific Vesicle Morphology and Associating Molecular Markers.Front Cell Dev Biol. 2021 Apr 27;9:662945. doi: 10.3389/fcell.2021.662945. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33987184 Free PMC article.
-
The genome of Spironucleus salmonicida highlights a fish pathogen adapted to fluctuating environments.PLoS Genet. 2014 Feb 6;10(2):e1004053. doi: 10.1371/journal.pgen.1004053. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24516394 Free PMC article.
-
Rac Regulates Giardia lamblia Encystation by Coordinating Cyst Wall Protein Trafficking and Secretion.mBio. 2016 Aug 23;7(4):e01003-16. doi: 10.1128/mBio.01003-16. mBio. 2016. PMID: 27555307 Free PMC article.
-
The multilayered interlaced network (MIN) in the sporoplasm of the microsporidium Anncaliia algerae is derived from Golgi.J Eukaryot Microbiol. 2013 Mar-Apr;60(2):166-78. doi: 10.1111/jeu.12019. Epub 2013 Jan 14. J Eukaryot Microbiol. 2013. PMID: 23316714 Free PMC article.
References
-
- Gillin FD, Boucher SE, Rossi SS, Reiner DS. Giardia lamblia: the roles of bile, lactic acid, and pH in the completion of the life cycle in vitro. Exp Parasitol. 1989;69:164–174. - PubMed
-
- Dacks JB, Field MC. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci. 2007;120:2977–2985. - PubMed
-
- Marti M, Regos A, Li Y, Schraner EM, Wild P, et al. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J Biol Chem. 2003;278:24837–24848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

