Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells
- PMID: 20386739
- PMCID: PMC2851565
- DOI: 10.1371/journal.pgen.1000898
Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells
Abstract
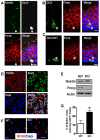
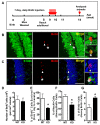
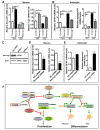
Fragile X syndrome (FXS), the most common form of inherited mental retardation, is caused by the loss of functional fragile X mental retardation protein (FMRP). FMRP is an RNA-binding protein that can regulate the translation of specific mRNAs. Adult neurogenesis, a process considered important for neuroplasticity and memory, is regulated at multiple molecular levels. In this study, we investigated whether Fmrp deficiency affects adult neurogenesis. We show that in a mouse model of fragile X syndrome, adult neurogenesis is indeed altered. The loss of Fmrp increases the proliferation and alters the fate specification of adult neural progenitor/stem cells (aNPCs). We demonstrate that Fmrp regulates the protein expression of several components critical for aNPC function, including CDK4 and GSK3beta. Dysregulation of GSK3beta led to reduced Wnt signaling pathway activity, which altered the expression of neurogenin1 and the fate specification of aNPCs. These data unveil a novel regulatory role for Fmrp and translational regulation in adult neurogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Penagarikano O, Mulle JG, Warren ST. The pathophysiology of fragile x syndrome. Annu Rev Genomics Hum Genet. 2007;8:109–129. - PubMed
-
- Sung YJ, Dolzhanskaya N, Nolin SL, Brown T, Currie JR, et al. The fragile X mental retardation protein FMRP binds elongation factor 1A mRNA and negatively regulates its translation in vivo. J Biol Chem. 2003;278:15669–15678. - PubMed
-
- Brennan FX, Albeck DS, Paylor R. Fmr1 knockout mice are impaired in a leverpress escape/avoidance task. Genes Brain Behav. 2006;5:467–471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

