P2Y2 nucleotide receptor-mediated responses in brain cells
- PMID: 20387013
- PMCID: PMC3086510
- DOI: 10.1007/s12035-010-8115-7
P2Y2 nucleotide receptor-mediated responses in brain cells
Abstract
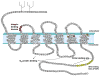
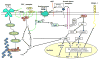
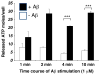
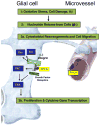
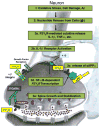
Acute inflammation is important for tissue repair; however, chronic inflammation contributes to neurodegeneration in Alzheimer's disease (AD) and occurs when glial cells undergo prolonged activation. In the brain, stress or damage causes the release of nucleotides and activation of the G(q) protein-coupled P2Y(2) nucleotide receptor subtype (P2Y(2)R) leading to pro-inflammatory responses that can protect neurons from injury, including the stimulation and recruitment of glial cells. P2Y(2)R activation induces the phosphorylation of the epidermal growth factor receptor (EGFR), a response dependent upon the presence of a SH3 binding domain in the intracellular C terminus of the P2Y(2)R that promotes Src binding and transactivation of EGFR, a pathway that regulates the proliferation of cortical astrocytes. Other studies indicate that P2Y(2)R activation increases astrocyte migration. P2Y(2)R activation by UTP increases the expression in astrocytes of alpha(V)beta(3/5) integrins that bind directly to the P2Y(2)R via an Arg-Gly-Asp (RGD) motif in the first extracellular loop of the P2Y(2)R, an interaction required for G(o) and G(12) protein-dependent astrocyte migration. In rat primary cortical neurons (rPCNs) P2Y(2)R expression is increased by stimulation with interleukin-1beta (IL-1beta), a pro-inflammatory cytokine whose levels are elevated in AD, in part due to nucleotide-stimulated release from glial cells. Other results indicate that oligomeric beta-amyloid peptide (Abeta(1-42)), a contributor to AD, increases nucleotide release from astrocytes, which would serve to activate upregulated P2Y(2)Rs in neurons. Data with rPCNs suggest that P2Y(2)R upregulation by IL-1beta and subsequent activation by UTP are neuroprotective, since this increases the non-amyloidogenic cleavage of amyloid precursor protein. Furthermore, activation of IL-1beta-upregulated P2Y(2)Rs in rPCNs increases the phosphorylation of cofilin, a cytoskeletal protein that stabilizes neurite outgrowths. Thus, activation of pro-inflammatory P2Y(2)Rs in glial cells can promote neuroprotective responses, suggesting that P2Y(2)Rs represent a novel pharmacological target in neurodegenerative and other pro-inflammatory diseases.
Figures
Similar articles
-
Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor.J Neurochem. 2009 Jun;109(5):1300-10. doi: 10.1111/j.1471-4159.2009.06048.x. Epub 2009 Mar 20. J Neurochem. 2009. PMID: 19317852 Free PMC article.
-
Molecular determinants of P2Y2 nucleotide receptor function: implications for proliferative and inflammatory pathways in astrocytes.Mol Neurobiol. 2005;31(1-3):169-83. doi: 10.1385/MN:31:1-3:169. Mol Neurobiol. 2005. PMID: 15953819 Review.
-
P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells.J Biol Chem. 2010 Mar 5;285(10):7545-55. doi: 10.1074/jbc.M109.078170. Epub 2010 Jan 11. J Biol Chem. 2010. PMID: 20064929 Free PMC article.
-
The P2Y2 nucleotide receptor interacts with alphav integrins to activate Go and induce cell migration.J Biol Chem. 2005 Nov 25;280(47):39050-7. doi: 10.1074/jbc.M504819200. Epub 2005 Sep 26. J Biol Chem. 2005. PMID: 16186116
-
Neuroprotective roles of the P2Y(2) receptor.Purinergic Signal. 2012 Sep;8(3):559-78. doi: 10.1007/s11302-012-9307-6. Epub 2012 Apr 14. Purinergic Signal. 2012. PMID: 22528682 Free PMC article. Review.
Cited by
-
Infection by Toxoplasma gondii, a severe parasite in neonates and AIDS patients, causes impaired anion secretion in airway epithelia.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):4435-40. doi: 10.1073/pnas.1503474112. Epub 2015 Mar 23. Proc Natl Acad Sci U S A. 2015. PMID: 25831498 Free PMC article.
-
P2Y Receptors in Synaptic Transmission and Plasticity: Therapeutic Potential in Cognitive Dysfunction.Neural Plast. 2016;2016:1207393. doi: 10.1155/2016/1207393. Epub 2016 Mar 16. Neural Plast. 2016. PMID: 27069691 Free PMC article. Review.
-
Pathophysiology of astroglial purinergic signalling.Purinergic Signal. 2012 Sep;8(3):629-57. doi: 10.1007/s11302-012-9300-0. Epub 2012 May 1. Purinergic Signal. 2012. PMID: 22544529 Free PMC article. Review.
-
Protein kinase C {delta}-specific activity reporter reveals agonist-evoked nuclear activity controlled by Src family of kinases.J Biol Chem. 2010 Dec 31;285(53):41896-910. doi: 10.1074/jbc.M110.184028. Epub 2010 Oct 19. J Biol Chem. 2010. PMID: 20959447 Free PMC article.
-
Cellular signaling pathways in the nervous system activated by various mechanical and electromagnetic stimuli.Front Mol Neurosci. 2024 Oct 4;17:1427070. doi: 10.3389/fnmol.2024.1427070. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39430293 Free PMC article. Review.
References
-
- Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer’s disease. Curr Opin Lipidol. 2002;13:289–294. - PubMed
-
- Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J Nutr Biochem. 2002;13:444–461. - PubMed
-
- Mattson MP. Oxidative stress, perturbed calcium homeostasis, and immune dysfunction in Alzheimer’s disease. J Neurovirol. 2002;8:539–550. - PubMed
-
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer’s disease. Free Radic Biol Med. 2002;33:620–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

