Total synthesis of (+)-complanadine A using an iridium-catalyzed pyridine C-H functionalization
- PMID: 20387895
- PMCID: PMC2867450
- DOI: 10.1021/ja101893b
Total synthesis of (+)-complanadine A using an iridium-catalyzed pyridine C-H functionalization
Abstract
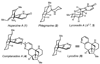
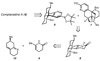
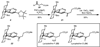
The total synthesis of the Lycopodium alkaloid complanadine A, which is an unsymmetrical dimer of lycodine, was achieved by exploiting a common tetracyclic precursor. Key to the success of the synthesis was the development of a late-stage site-selective C-H functionalization of a pyridine moiety to arrive at a key boronic ester intermediate.
Figures
Similar articles
-
One-Carbon Insertion and Polarity Inversion Enabled a Pyrrole Strategy to the Total Syntheses of Pyridine-Containing Lycopodium Alkaloids: Complanadine A and Lycodine.J Am Chem Soc. 2021 Oct 13;143(40):16383-16387. doi: 10.1021/jacs.1c08626. Epub 2021 Sep 27. J Am Chem Soc. 2021. PMID: 34570487 Free PMC article.
-
Synthetic studies on pseudo-dimeric Lycopodium alkaloids: total synthesis of complanadine B.Angew Chem Int Ed Engl. 2013 Feb 4;52(6):1726-30. doi: 10.1002/anie.201208571. Epub 2013 Jan 10. Angew Chem Int Ed Engl. 2013. PMID: 23307758 Free PMC article. No abstract available.
-
Synthesis of (+)-complanadine A, an inducer of neurotrophic factor excretion.J Am Chem Soc. 2010 May 5;132(17):5924-5. doi: 10.1021/ja101956x. J Am Chem Soc. 2010. PMID: 20387896
-
Lycopodium alkaloids: isolation and asymmetric synthesis.Top Curr Chem. 2012;309:1-31. doi: 10.1007/128_2011_126. Top Curr Chem. 2012. PMID: 21452079 Review.
-
Reaction strategies for the meta-selective functionalization of pyridine through dearomatization.Mol Divers. 2025 Feb;29(1):849-869. doi: 10.1007/s11030-024-10861-5. Epub 2024 Apr 22. Mol Divers. 2025. PMID: 38647989 Review.
Cited by
-
Toward a unified approach for the lycopodines: synthesis of 10-hydroxylycopodine, deacetylpaniculine, and paniculine.Org Lett. 2013 Feb 15;15(4):736-9. doi: 10.1021/ol303272w. Epub 2013 Feb 5. Org Lett. 2013. PMID: 23384410 Free PMC article.
-
Direct C7 Functionalization of Tryptophan. Synthesis of Methyl (S)-2-((tert-Butoxycarbonyl)amino)-3-(7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl)propanoate.Organic Synth. 2015;92:373-385. doi: 10.15227/orgsyn.092.0373. Organic Synth. 2015. PMID: 26839440 Free PMC article.
-
Unconventional Macrocyclizations in Natural Product Synthesis.ACS Cent Sci. 2020 Nov 25;6(11):1869-1889. doi: 10.1021/acscentsci.0c00599. Epub 2020 Sep 21. ACS Cent Sci. 2020. PMID: 33274267 Free PMC article. Review.
-
Divergent total syntheses of ITHQ-type bis-β-carboline alkaloids by regio-selective formal aza-[4 + 2] cycloaddition and late-stage C-H functionalization.Chem Sci. 2023 Sep 13;14(37):10353-10359. doi: 10.1039/d3sc03722c. eCollection 2023 Sep 27. Chem Sci. 2023. PMID: 37772099 Free PMC article.
-
Advancing Total Synthesis Through Skeletal Editing.Acc Chem Res. 2025 May 6;58(9):1392-1406. doi: 10.1021/acs.accounts.5c00030. Epub 2025 Apr 10. Acc Chem Res. 2025. PMID: 40209068 Free PMC article.
References
-
-
For a pertinent review, see: Ma X, Gang DR. Nat. Prod. Rep. 2004;21:752–772. Ayer WA, Trofinov LS. Lycopodium Alkaloids. San Diego: Academic Press; 1994. Hudlicky T, Reed JW. The Way of Synthesis. 1st Ed. Weinheim: Wiley-VCH; 2007. Lycopodium Alkaloids; pp. 573–602..
-
-
- Liang YQ, Tang XC. Neurosci. Lett. 2004;361:56–59. - PubMed
-
- Jiang HL, Luo XM, Bai DL. Curr. Med. Chem. 2003;10:2231–2252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources