Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions
- PMID: 20387898
- PMCID: PMC2908497
- DOI: 10.1021/ja100739h
Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions
Abstract
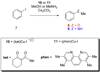
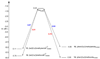
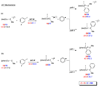
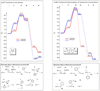
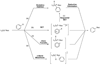
Computational investigations of ligand-directed selectivities in Ullmann-type coupling reactions of methanol and methylamine with iodobenzene by beta-diketone- and 1,10-phenanthroline-ligated Cu(I) complexes are reported. Density functional theory calculations using several functionals were performed on both the nucleophile formation and aryl halide activation steps of these reactions. The origin of ligand-directed selectivities in N- versus O-arylation reactions as described in a previous publication (J. Am. Chem. Soc. 2007, 129, 3490-3491) were studied and explained. The selectivities observed experimentally are derived not from initial Cu(I)(nucleophile) complex formation but from the subsequent steps involving aryl halide activation. The arylation may occur via single-electron transfer (SET) or iodine atom transfer (IAT), depending on the electron-donating abilities of the ligand and nucleophile. Mechanisms involving either oxidative addition/reductive elimination or sigma-bond metathesis are disfavored. SET mechanisms are favored in reactions promoted by the beta-diketone ligand; N-arylation is predicted to be favored in these cases, in agreement with experimental results. The phenanthroline ligand promotes O-arylation reactions via IAT mechanisms in preference to N-arylation reactions, which occur via SET mechanisms; this result is also in agreement with experimental results.
Figures



References
-
- Ullmann F, Bielecki J. Chem. Ber. 1901;34:2174–2185.
- Ullmann F. Chem. Ber. 1903;36:2382–2384.
- Ullmann F, Sponagel P. Chem. Ber. 1905;38:2211–2212.
- Goldberg I. Chem. Ber. 1906;39:1691–1692.
-
- Shafir A, Buchwald SL. J. Am. Chem. Soc. 2006;128:8742–8743. - PubMed
-
- de Lange B, Lambers-Verstappen MH, Schmieder-van de Vondervoort L, Sereinig N, de Rijk R, de Vries AHM, de Vries JG. Synlett. 2006:3105–3109.
-
- Goodbrand HB, Hu NX. J. Org. Chem. 1999;64:670–674.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

