Lysosomal function in macromolecular homeostasis and bioenergetics in Parkinson's disease
- PMID: 20388210
- PMCID: PMC2867960
- DOI: 10.1186/1750-1326-5-14
Lysosomal function in macromolecular homeostasis and bioenergetics in Parkinson's disease
Abstract
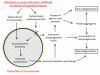
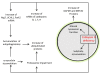
The pathological changes occurring in Parkinson's and several other neurodegenerative diseases are complex and poorly understood, but all clearly involve protein aggregation. Also frequently appearing in neurodegeneration is mitochondrial dysfunction which may precede, coincide or follow protein aggregation. These observations led to the concept that protein aggregation and mitochondrial dysfunction either arise from the same etiological factors or are interactive. Understanding the mechanisms and regulation of processes that lead to protein aggregation or mitochondrial dysfunction may therefore contribute to the design of better therapeutics. Clearance of protein aggregates and dysfunctional organelles is dependent on macroautophagy which is the process through which aged or damaged proteins and organelles are first degraded by the lysosome and then recycled. The macroautophagy-lysosomal pathway is essential for maintaining protein and energy homeostasis. Not surprisingly, failure of the lysosomal system has been implicated in diseases that have features of protein aggregation and mitochondrial dysfunction. This review summarizes 3 major topics: 1) the current understanding of Parkinson's disease pathogenesis in terms of accumulation of damaged proteins and reduction of cellular bioenergetics; 2) evolving insights into lysosomal function and biogenesis and the accumulating evidence that lysosomal dysfunction may cause or exacerbate Parkinsonian pathology and finally 3) the possibility that enhancing lysosomal function may provide a disease modifying therapy.
Figures
References
-
- Fuertes G, Martin de Llano JJ, Villarroya A, Rivett AJ, Knecht E. Changes in the proteolytic activities of proteasomes and lysosomes in human fibroblasts produced by serum withdrawal, amino-acid deprivation and confluent conditions. Biochem J. 2003;375:75–86. doi: 10.1042/BJ20030282. - DOI - PMC - PubMed
-
- Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4:590–599. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

