Co-expression of Skp and FkpA chaperones improves cell viability and alters the global expression of stress response genes during scFvD1.3 production
- PMID: 20388215
- PMCID: PMC2868799
- DOI: 10.1186/1475-2859-9-22
Co-expression of Skp and FkpA chaperones improves cell viability and alters the global expression of stress response genes during scFvD1.3 production
Abstract
Background: The overexpression of scFv antibody fragments in the periplasmic space of Escherichia coli frequently results in extensive protein misfolding and loss of cell viability. Although protein folding factors such as Skp and FkpA are often exploited to restore the solubility and functionality of recombinant protein products, their exact impact on cellular metabolism during periplasmic antibody fragment expression is not clearly understood. In this study, we expressed the scFvD1.3 antibody fragment in E. coli BL21 and evaluated the overall physiological and global gene expression changes upon Skp or FkpA co-expression.
Results: The periplasmic expression of scFvD1.3 led to a rapid accumulation of insoluble scFvD1.3 proteins and a decrease in cell viability. The co-expression of Skp and FkpA improved scFvD1.3 solubility and cell viability in a dosage-dependent manner. Through mutagenesis experiments, it was found that only the chaperone activity of FkpA, not the peptidyl-prolyl isomerase (PPIase) activity, is required for the improvement in cell viability. Global gene expression analysis of the scFvD1.3 cells over the chaperone-expressing cells showed a clear up-regulation of genes involved in heat-shock and misfolded protein stress responses. These included genes of the major HSP70 DnaK chaperone family and key proteases belonging to the Clp and Lon protease systems. Other metabolic gene expression trends include: (1) the differential regulation of several energy metabolic genes, (2) down-regulation of the central metabolic TCA cycle and transport genes, and (3) up-regulation of ribosomal genes.
Conclusions: The simultaneous activation of multiple stress related and other metabolic genes may constitute the stress response to protein misfolding in the scFvD1.3 cells. These gene expression information could prove to be valuable for the selection and construction of reporter contructs to monitor the misfolded protein stress response during antibody fragment production.
Figures



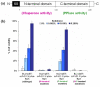
References
-
- Viti F, Tarli L, Giovannoni L, Zardi L, Neri D. Increased binding affinity and valence of recombinant antibody fragments lead to improved targeting of tumoral angiogenesis. Cancer Res. 1999;59:347–352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

