Cyclooxygenase-2 expression in oligodendrocytes increases sensitivity to excitotoxic death
- PMID: 20388219
- PMCID: PMC2873241
- DOI: 10.1186/1742-2094-7-25
Cyclooxygenase-2 expression in oligodendrocytes increases sensitivity to excitotoxic death
Abstract
Background: We previously found that cyclooxygenase 2 (COX-2) was expressed in dying oligodendrocytes at the onset of demyelination in the Theiler's murine encephalomyelitis virus-induced demyelinating disease (TMEV-IDD) model of multiple sclerosis (MS) (Carlson et al. J.Neuroimmunology 2006, 149:40). This suggests that COX-2 may contribute to death of oligodendrocytes.
Objective: The goal of this study was to examine whether COX-2 contributes to excitotoxic death of oligodendrocytes and potentially contributes to demyelination.
Methods: The potential link between COX-2 and oligodendrocyte death was approached using histopathology of MS lesions to examine whether COX-2 was expressed in dying oligodendrocytes. COX-2 inhibitors were examined for their ability to limit demyelination in the TMEV-IDD model of MS and to limit excitotoxic death of oligodendrocytes in vitro. Genetic manipulation of COX-2 expression was used to determine whether COX-2 contributes to excitotoxic death of oligodendrocytes. A transgenic mouse line was generated that overexpressed COX-2 in oligodendrocytes. Oligodendrocyte cultures derived from these transgenic mice were used to examine whether increased expression of COX-2 enhanced the vulnerability of oligodendrocytes to excitotoxic death. Oligodendrocytes derived from COX-2 knockout mice were evaluated to determine if decreased COX-2 expression promotes a greater resistance to excitotoxic death.
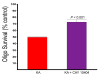
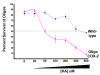
Results: COX-2 was expressed in dying oligodendrocytes in MS lesions. COX-2 inhibitors limited demyelination in the TMEV-IDD model of MS and protected oligodendrocytes against excitotoxic death in vitro. COX-2 expression was increased in wild-type oligodendrocytes following treatment with Kainic acid (KA). Overexpression of COX-2 in oligodendrocytes increased the sensitivity of oligodendrocytes to KA-induced excitotoxic death eight-fold compared to wild-type. Conversely, oligodendrocytes prepared from COX-2 knockout mice showed a significant decrease in sensitivity to KA induced death.
Conclusions: COX-2 expression was associated with dying oligodendrocytes in MS lesions and appeared to increase excitotoxic death of oligodendrocytes in culture. An understanding of how COX-2 expression influences oligodendrocyte death leading to demyelination may have important ramifications for future treatments for MS.
Figures




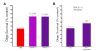
Similar articles
-
The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis.J Neuroimmunol. 2006 May;174(1-2):21-31. doi: 10.1016/j.jneuroim.2006.01.008. Epub 2006 Mar 3. J Neuroimmunol. 2006. PMID: 16516308
-
Comparison of Reported Spinal Cord Lesions in Progressive Multiple Sclerosis with Theiler's Murine Encephalomyelitis Virus Induced Demyelinating Disease.Int J Mol Sci. 2019 Feb 25;20(4):989. doi: 10.3390/ijms20040989. Int J Mol Sci. 2019. PMID: 30823515 Free PMC article.
-
Double-edged effects of tamoxifen-in-oil-gavage on an infectious murine model for multiple sclerosis.Brain Pathol. 2021 Nov;31(6):e12994. doi: 10.1111/bpa.12994. Epub 2021 Jun 17. Brain Pathol. 2021. PMID: 34137105 Free PMC article.
-
Antibody-Mediated Oligodendrocyte Remyelination Promotes Axon Health in Progressive Demyelinating Disease.Mol Neurobiol. 2016 Oct;53(8):5217-28. doi: 10.1007/s12035-015-9436-3. Epub 2015 Sep 26. Mol Neurobiol. 2016. PMID: 26409478 Free PMC article. Review.
-
Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review.
Cited by
-
Should We Consider Neurodegeneration by Itself or in a Triangulation with Neuroinflammation and Demyelination? The Example of Multiple Sclerosis and Beyond.Int J Mol Sci. 2024 Nov 25;25(23):12637. doi: 10.3390/ijms252312637. Int J Mol Sci. 2024. PMID: 39684351 Free PMC article. Review.
-
Oligodendrocyte-microglia cross-talk in the central nervous system.Immunology. 2014 Mar;141(3):302-13. doi: 10.1111/imm.12163. Immunology. 2014. PMID: 23981039 Free PMC article. Review.
-
PET imaging in multiple sclerosis.J Neuroimmune Pharmacol. 2014 Sep;9(4):468-82. doi: 10.1007/s11481-014-9544-2. Epub 2014 May 9. J Neuroimmune Pharmacol. 2014. PMID: 24809810 Review.
-
Ketogenic diets attenuate cyclooxygenase and lipoxygenase gene expression in multiple sclerosis.EBioMedicine. 2018 Oct;36:293-303. doi: 10.1016/j.ebiom.2018.08.057. Epub 2018 Oct 3. EBioMedicine. 2018. PMID: 30292675 Free PMC article. Clinical Trial.
-
Arachidonic acid-derived lipid mediators in multiple sclerosis pathogenesis: fueling or dampening disease progression?J Neuroinflammation. 2024 Jan 17;21(1):21. doi: 10.1186/s12974-023-02981-w. J Neuroinflammation. 2024. PMID: 38233951 Free PMC article. Review.
References
-
- Weinshenker BG, Ebers GC. The natural history of multiple sclerosis. Can J Neurol Sci. 1987;14:255–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

