Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins
- PMID: 20388661
- PMCID: PMC2879769
- DOI: 10.1104/pp.110.155424
Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins
Abstract
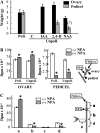
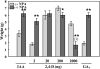
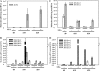
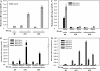
Fruit-set in tomato (Solanum lycopersicum) depends on gibberellins and auxins (GAs). Here, we show, using the cv MicroTom, that application of N-1-naphthylphthalamic acid (NPA; an inhibitor of auxin transport) to unpollinated ovaries induced parthenocarpic fruit-set, associated with an increase of indole-3-acetic acid (IAA) content, and that this effect was negated by paclobutrazol (an inhibitor of GA biosynthesis). NPA-induced ovaries contained higher content of GA(1) (an active GA) and transcripts of GA biosynthetic genes (SlCPS, SlGA20ox1, and -2). Interestingly, application of NPA to pollinated ovaries prevented their growth, potentially due to supraoptimal IAA accumulation. Plant decapitation and inhibition of auxin transport by NPA from the apical shoot also induced parthenocarpic fruit growth of unpollinated ovaries. Application of IAA to the severed stump negated the plant decapitation effect, indicating that the apical shoot prevents unpollinated ovary growth through IAA transport. Parthenocarpic fruit growth induced by plant decapitation was associated with high levels of GA(1) and was counteracted by paclobutrazol treatment. Plant decapitation also produced changes in transcript levels of genes encoding enzymes of GA biosynthesis (SlCPS and SlGA20ox1) in the ovary, quite similar to those found in NPA-induced fruits. All these results suggest that auxin can have opposing effects on fruit-set, either inducing (when accumulated in the ovary) or repressing (when transported from the apical shoot) that process, and that GAs act as mediators in both cases. The effect of NPA application and decapitation on fruit-set induction was also observed in MicroTom lines bearing introgressed DWARF and SELF-PRUNING wild-type alleles.
Figures

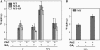

References
-
- Abad M, Monteiro AA. (1989) The use of auxins for the production of greenhouse tomatoes in mild-winter conditions: a review. Sci Hortic 38: 167–192
-
- Bangerth F. (1989) Dominance among fruits/sinks and the search for a correlative signal. Physiol Plant 76: 608–614
-
- Bangerth F, Li CJ, Gruber J. (2000) Mutual interaction of auxin and cytokinins in regulating correlative dominance. Plant Growth Regul 32: 205–217
-
- Banuelos GS, Bangerth F, Marschner H. (1987) Relationship between polar basipetal auxin transport and acropetal Ca2+ transport into tomato fruits. Physiol Plant 71: 321–327
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

