Arabinogalactan-proteins: key regulators at the cell surface?
- PMID: 20388666
- PMCID: PMC2879789
- DOI: 10.1104/pp.110.156000
Arabinogalactan-proteins: key regulators at the cell surface?
Erratum in
- Plant Physiol. 2010 Oct;154(2):1012
Figures
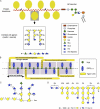

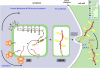
References
-
- Andème-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A. (2002) The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta 215: 949–958 - PubMed
-
- Andersson-Gunnerås S, Mellerowicz EJ, Love J, Segerman B, Ohmiya Y, Coutinho PM, Nilsson P, Henrissat B, Moritz T, Sundberg B. (2006) Biosynthesis of cellulose-enriched tension wood in Populus: global analysis of transcripts and metabolites identifies biochemical and developmental regulators in secondary wall biosynthesis. Plant J 45: 144–165 - PubMed
-
- Bacic A, Churms SC, Stephen AM, Cohen PB, Fincher GB. (1987) Fine structure of the arabinogalactan-protein from Lolium multiflorum. Carbohydr Res 162: 85–93
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

