A pollen-specific RALF from tomato that regulates pollen tube elongation
- PMID: 20388667
- PMCID: PMC2879774
- DOI: 10.1104/pp.110.155457
A pollen-specific RALF from tomato that regulates pollen tube elongation
Abstract
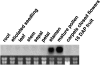
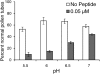
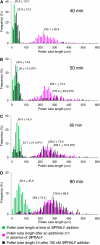
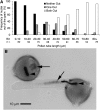
Rapid Alkalinization Factors (RALFs) are plant peptides that rapidly increase the pH of plant suspension cell culture medium and inhibit root growth. A pollen-specific tomato (Solanum lycopersicum) RALF (SlPRALF) has been identified. The SlPRALF gene encodes a preproprotein that appears to be processed and released from the pollen tube as an active peptide. A synthetic SlPRALF peptide based on the putative active peptide did not affect pollen hydration or viability but inhibited the elongation of normal pollen tubes in an in vitro growth system. Inhibitory effects of SlPRALF were detectable at concentrations as low as 10 nm, and complete inhibition was observed at 1 mum peptide. At least 10-fold higher levels of alkSlPRALF, which lacks disulfide bonds, were required to see similar effects. A greater effect of peptide was observed in low-pH-buffered medium. Inhibition of pollen tube elongation was reversible if peptide was removed within 15 min of exposure. Addition of 100 nm SlPRALF to actively growing pollen tubes inhibited further elongation until tubes were 40 to 60 mum in length, after which pollen tubes became resistant to the peptide. The onset of resistance correlated with the timing of the exit of the male germ unit from the pollen grain into the tube. Thus, exogenous SlPRALF acts as a negative regulator of pollen tube elongation within a specific developmental window.
Figures



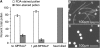

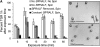
References
-
- Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA, Simmons MP, Bedinger P, Goff SA, Ringli C, et al. (2003) Whole-genome comparison of leucine-rich repeat extensins in Arabidopsis and rice: a conserved family of cell wall proteins form a vegetative and a reproductive clade. Plant Physiol 131: 1313–1326 - PMC - PubMed
-
- Boller T. (2005) Peptide signalling in plant development and self/non-self perception. Curr Opin Cell Biol 17: 116–122 - PubMed
-
- Brukhin V, Gonzalez HN, Chevalier C, Mouras A. (2003) Flower development schedule in tomato Lycopersicon esculentum cv. Sweet Cherry. Sex Plant Reprod 15: 311–320
-
- Chen YF, Matsubayashi Y, Sakagami Y. (2000) Peptide growth factor phytosulfokine-alpha contributes to the pollen population effect. Planta 211: 752–755 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

