DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth
- PMID: 20388669
- PMCID: PMC2879792
- DOI: 10.1104/pp.110.154963
DNA is taken up by root hairs and pollen, and stimulates root and pollen tube growth
Abstract
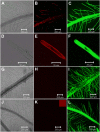
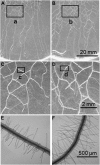
Phosphorus (P) enters roots as inorganic phosphate (P(i)) derived from organic and inorganic P compounds in the soil. Nucleic acids can support plant growth as the sole source of P in axenic culture but are thought to be converted into P(i) by plant-derived nucleases and phosphatases prior to uptake. Here, we show that a nuclease-resistant analog of DNA is taken up by plant cells. Fluorescently labeled S-DNA of 25 bp, which is protected against enzymatic breakdown by its phosphorothioate backbone, was taken up and detected in root cells including root hairs and pollen tubes. These results indicate that current views of plant P acquisition may have to be revised to include uptake of DNA into cells. We further show that addition of DNA to P(i)-containing growth medium enhanced the growth of lateral roots and root hairs even though plants were P replete and had similar biomass as plants supplied with P(i) only. Exogenously supplied DNA increased length growth of pollen tubes, which were studied because they have similar elongated and polarized growth as root hairs. Our results indicate that DNA is not only taken up and used as a P source by plants, but ironically and independent of P(i) supply, DNA also induces morphological changes in roots similar to those observed with P limitation. This study provides, to our knowledge, first evidence that exogenous DNA could act nonspecifically as signaling molecules for root development.
Figures



Similar articles
-
Rates of exocytosis and endocytosis in Arabidopsis root hairs and pollen tubes.J Microsc. 2008 Aug;231(2):265-73. doi: 10.1111/j.1365-2818.2008.02031.x. J Microsc. 2008. PMID: 18778424
-
MARIS plays important roles in Arabidopsis pollen tube and root hair growth.J Integr Plant Biol. 2016 Nov;58(11):927-940. doi: 10.1111/jipb.12484. Epub 2016 Jun 23. J Integr Plant Biol. 2016. PMID: 27212106
-
Male gametophyte defective 4 encodes a rhamnogalacturonan II xylosyltransferase and is important for growth of pollen tubes and roots in Arabidopsis.Plant J. 2011 Feb;65(4):647-60. doi: 10.1111/j.1365-313X.2010.04452.x. Epub 2010 Dec 31. Plant J. 2011. PMID: 21288267
-
Vesicular trafficking, cytoskeleton and signalling in root hairs and pollen tubes.Trends Plant Sci. 2006 Dec;11(12):594-600. doi: 10.1016/j.tplants.2006.10.002. Epub 2006 Nov 7. Trends Plant Sci. 2006. PMID: 17092761 Review.
-
Quantitative cell biology of tip growth in moss.Plant Mol Biol. 2021 Nov;107(4-5):227-244. doi: 10.1007/s11103-021-01147-7. Epub 2021 Apr 6. Plant Mol Biol. 2021. PMID: 33825083 Free PMC article. Review.
Cited by
-
Green pesticide: Tapping to the promising roles of plant secreted small RNAs and responses towards extracellular DNA.Noncoding RNA Res. 2021 Feb 13;6(1):42-50. doi: 10.1016/j.ncrna.2021.02.001. eCollection 2021 Mar. Noncoding RNA Res. 2021. PMID: 33778217 Free PMC article. Review.
-
Exogenous RNAs for Gene Regulation and Plant Resistance.Int J Mol Sci. 2019 May 8;20(9):2282. doi: 10.3390/ijms20092282. Int J Mol Sci. 2019. PMID: 31072065 Free PMC article. Review.
-
The Multiple Facets of Plant-Fungal Interactions Revealed Through Plant and Fungal Secretomics.Front Plant Sci. 2020 Jan 8;10:1626. doi: 10.3389/fpls.2019.01626. eCollection 2019. Front Plant Sci. 2020. PMID: 31969889 Free PMC article. Review.
-
Calreticulin is required for calcium homeostasis and proper pollen tube tip growth in Petunia.Planta. 2017 May;245(5):909-926. doi: 10.1007/s00425-017-2649-0. Epub 2017 Jan 11. Planta. 2017. PMID: 28078426 Free PMC article.
-
Wheat Can Access Phosphorus From Algal Biomass as Quickly and Continuously as From Mineral Fertilizer.Front Plant Sci. 2021 Jan 28;12:631314. doi: 10.3389/fpls.2021.631314. eCollection 2021. Front Plant Sci. 2021. PMID: 33584779 Free PMC article.
References
-
- Anderson G. (1980) Assessing organic phosphorus in soils. Khasawneh F, Sample E, Kamprath E, , The Role of Phosphorus in Agriculture. American Society of Agronomy, Madison, WI, pp 411–432
-
- Bates TR, Lynch JP. (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19: 529–538
-
- Bieleski R. (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24: 225–252
-
- Bower C. (1945) Separation and identification of phytin and its derivatives from soils. Soil Sci 59: 277–285
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

