Mucin 21/epiglycanin modulates cell adhesion
- PMID: 20388707
- PMCID: PMC2898422
- DOI: 10.1074/jbc.M109.082875
Mucin 21/epiglycanin modulates cell adhesion
Abstract
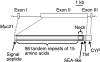
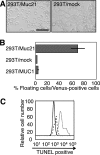
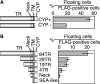
The molecular structure of mouse Mucin 21 (Muc21)/epiglycanin is proposed to have 98 tandem repeats of 15 amino acids and three exceptional repeats with 12 or 13 amino acids each, followed by a stem domain, a transmembrane domain, and a cytoplasmic tail. A cDNA of Muc21 having 84 tandem repeats of 15 amino acids was constructed and transfected using a Venus vector into HEK 293T cells. The fluorescent cells, which were considered to express Muc21, were nonadherent. This antiadhesion effect was lessened when constructs with smaller numbers of tandem repeats were used, suggesting that the tandem repeat domain plays a crucial role. Cells expressing Muc21 were significantly less adherent to each other and to extracellular matrix components than control cells. Antibody binding to the cell surface integrin subunits alpha5, alpha6, and beta1 was reduced in Muc21 transfectants in a tandem repeat-dependent manner, whereas equal amounts of proteins were detected by Western blot analysis. Muc21 was expressed as a large glycoprotein that was highly glycosylated with O-glycans at the cell surface, as detected by flow cytometry, Western blotting, and lectin blotting. Although at least a portion of Muc21 was glycosylated with sialylated glycans, removal of sialic acid did not influence the prevention of adhesion.
Figures




References
-
- Codington J. F., Linsley K. B., Jeanloz R. W., Irimura T., Osawa T. (1975) Carbohydr. Res. 40, 171–182 - PubMed
-
- Codington J. F., Sanford B. H., Jeanloz R. W. (1972) Biochemistry 11, 2559–2564 - PubMed
-
- Hauschka T. S., Weiss L., Holdridge B. A., Cudney T. L., Zumpft M., Planinsek J. A. (1971) J. Natl. Cancer Inst. 47, 343–359 - PubMed
-
- Klein G. (1951) Exp. Cell Res. 2, 291–294
-
- Friberg S., Jr. (1972) J. Natl. Cancer Inst. 48, 1463–1476 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

