Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase
- PMID: 20388710
- PMCID: PMC2885237
- DOI: 10.1074/jbc.M110.119248
Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase
Abstract
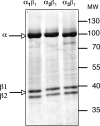
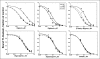
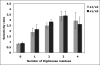
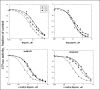
There are four isoforms of the alpha subunit (alpha1-4) and three isoforms of the beta subunit (beta1-3) of Na,K-ATPase, with distinct tissue-specific distribution and physiological functions. alpha2 is thought to play a key role in cardiac and smooth muscle contraction and be an important target of cardiac glycosides. An alpha2-selective cardiac glycoside could provide important insights into physiological and pharmacological properties of alpha2. The isoform selectivity of a large number of cardiac glycosides has been assessed utilizing alpha1beta1, alpha2beta1, and alpha3beta1 isoforms of human Na,K-ATPase expressed in Pichia pastoris and the purified detergent-soluble isoform proteins. Binding affinities of the digitalis glycosides, digoxin, beta-methyl digoxin, and digitoxin show moderate but highly significant selectivity (up to 4-fold) for alpha2/alpha3 over alpha1 (K(D) alpha1 > alpha2 = alpha3). By contrast, ouabain shows moderate selectivity ( approximately 2.5-fold) for alpha1 over alpha2 (K(D) alpha1 <or= alpha3 < alpha2). Binding affinities for the three isoforms of digoxigenin, digitoxigenin, and all other aglycones tested are indistinguishable (K(D) alpha1 = alpha3 = alpha2), showing that the sugar determines isoform selectivity. Selectivity patterns for inhibition of Na,K-ATPase activity of the purified isoform proteins are consistent with binding selectivities, modified somewhat by different affinities of K(+) ions for antagonizing cardiac glycoside binding on the three isoforms. The mechanistic insight on the role of the sugars is strongly supported by a recent structure of Na,K-ATPase with bound ouabain, which implies that aglycones of cardiac glycosides cannot discriminate between isoforms. In conclusion, several digitalis glycosides, but not ouabain, are moderately alpha2-selective. This supports a major role of alpha2 in cardiac contraction and cardiotonic effects of digitalis glycosides.
Figures

Similar articles
-
Cardiac glycosides induced toxicity in human cells expressing α1-, α2-, or α3-isoforms of Na-K-ATPase.Am J Physiol Cell Physiol. 2015 Jul 15;309(2):C126-35. doi: 10.1152/ajpcell.00089.2015. Am J Physiol Cell Physiol. 2015. PMID: 25994790
-
Isoform specificity of cardiac glycosides binding to human Na+,K+-ATPase alpha1beta1, alpha2beta1 and alpha3beta1.Eur J Pharmacol. 2009 Nov 10;622(1-3):7-14. doi: 10.1016/j.ejphar.2009.08.039. Epub 2009 Sep 12. Eur J Pharmacol. 2009. PMID: 19751721 Free PMC article.
-
The cardiac sodium pump: structure and function.Basic Res Cardiol. 2002;97 Suppl 1:I19-24. doi: 10.1007/s003950200024. Basic Res Cardiol. 2002. PMID: 12479229 Review.
-
Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site.Am J Physiol Regul Integr Comp Physiol. 2006 Mar;290(3):R524-8. doi: 10.1152/ajpregu.00838.2005. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16467499 Review.
-
The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation.Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):15845-50. doi: 10.1073/pnas.0507358102. Epub 2005 Oct 21. Proc Natl Acad Sci U S A. 2005. PMID: 16243970 Free PMC article.
Cited by
-
Epi-reevesioside F inhibits Na+/K+-ATPase, causing cytosolic acidification, Bak activation and apoptosis in glioblastoma.Oncotarget. 2015 Sep 15;6(27):24032-46. doi: 10.18632/oncotarget.4429. Oncotarget. 2015. PMID: 26125228 Free PMC article.
-
Short-Term Mild Hypoxia Modulates Na,K-ATPase to Maintain Membrane Electrogenesis in Rat Skeletal Muscle.Int J Mol Sci. 2022 Oct 6;23(19):11869. doi: 10.3390/ijms231911869. Int J Mol Sci. 2022. PMID: 36233169 Free PMC article.
-
Digitoxin analogues with improved anticytomegalovirus activity.ACS Med Chem Lett. 2014 Jan 25;5(4):395-9. doi: 10.1021/ml400529q. eCollection 2014 Apr 10. ACS Med Chem Lett. 2014. PMID: 24900847 Free PMC article.
-
Digoxin derivatives with selectivity for the α2β3 isoform of Na,K-ATPase potently reduce intraocular pressure.Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13723-8. doi: 10.1073/pnas.1514569112. Epub 2015 Oct 19. Proc Natl Acad Sci U S A. 2015. PMID: 26483500 Free PMC article.
-
Physiologically Based Pharmacokinetic Modeling to Assess Ritonavir-Digoxin Interactions and Recommendations for Co-Administration Regimens.Pharm Res. 2024 Nov;41(11):2199-2212. doi: 10.1007/s11095-024-03789-w. Epub 2024 Nov 18. Pharm Res. 2024. PMID: 39557814
References
-
- Schoner W., Scheiner-Bobis G. (2007) Am. J. Physiol. Cell Physiol. 293, C509–C536 - PubMed
-
- Xie Z., Askari A. (2002) Eur. J. Biochem. 269, 2434–2439 - PubMed
-
- Li Z., Xie Z. (2009) Pflugers Arch. 457, 635–644 - PubMed
-
- Dvela M., Rosen H., Feldmann T., Nesher M., Lichtstein D. (2007) Pathophysiology 14, 159–166 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

