Stem cells as vectors for antitumour therapy
- PMID: 20388765
- PMCID: PMC3401681
- DOI: 10.1136/thx.2009.128025
Stem cells as vectors for antitumour therapy
Abstract
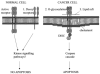
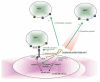
Recent research suggests that mesenchymal stem cells (MSCs) are able to migrate specifically to tumours and their metastases throughout the body. This has led to considerable excitement about the possibility of modifying these cells to express anticancer molecules and using them as specific targeted anticancer agents. However, there are concerns that systemically delivered MSCs may have non-desirable effects, and there are also many unanswered questions including the mechanism of tumour homing. This review investigates the different MSC-delivered anticancer agents, addresses the questions and concerns, and tries to place this potential therapy in future cancer management.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Bonnet D. Biology of human bone marrow stem cells. Clin Exp Med. 2003;3:140–9. - PubMed
-
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. - PubMed
-
- Ishii G, Sangai T, Oda T, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232–40. - PubMed
-
- Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
