Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection
- PMID: 20388791
- PMCID: PMC2856125
- DOI: 10.1158/0008-5472.CAN-09-3379
Tumor site-specific silencing of NF-kappaB p65 by targeted hollow gold nanosphere-mediated photothermal transfection
Abstract
NF-kappaB transcription factor is a critical regulator of the expression of genes involved in tumor formation and progression. Successful RNA interference (RNAi) therapeutics targeting NF-kappaB is challenged by small interfering RNA (siRNA) delivery systems, which can render targeted in vivo delivery, efficient endolysosomal escape, and dynamic control over activation of RNAi. Here, we report near-IR (NIR) light-inducible NF-kappaB downregulation through folate receptor-targeted hollow gold nanospheres carrying siRNA recognizing NF-kappaB p65 subunit. Using micro-positron emission tomography/computed tomography imaging, the targeted nanoconstructs exhibited significantly higher tumor uptake in nude mice bearing HeLa cervical cancer xenografts than nontargeted nanoparticles following i.v. administration. Mediated by hollow gold nanospheres, controllable cytoplasmic delivery of siRNA was obtained on NIR light irradiation through photothermal effect. Efficient downregulation of NF-kappaB p65 was achieved only in tumors irradiated with NIR light but not in nonirradiated tumors grown in the same mice. Liver, spleen, kidney, and lung were not affected by the treatments, in spite of significant uptake of the siRNA nanoparticles in these organs. We term this mode of action "photothermal transfection." Combined treatments with p65 siRNA photothermal transfection and irinotecan caused substantially enhanced tumor apoptosis and significant tumor growth delay compared with other treatment regimens. Therefore, photothermal transfection of NF-kappaB p65 siRNA could effectively sensitize the tumor to chemotherapeutic agents. Because NIR light can penetrate the skin and be delivered with high spatiotemporal control, therapeutic RNAi may benefit from this novel transfection strategy while avoiding unwanted side effect.
(c)2010 AACR.
Figures
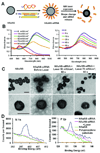


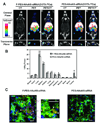
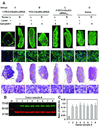
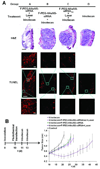
References
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
-
- Medina-Kauwe LK, Xie J, Hamm-Alvarez S. Intracellular trafficking of nonviral vectors. Gene Ther. 2005;12:1734–1751. - PubMed
-
- Boe S, Longva AS, Hovig E. Evaluation of various polyethylenimine formulations for light-controlled gene silencing using small interfering RNA molecules. Oligonucleotides. 2008;18:123–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

