The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice
- PMID: 20389022
- PMCID: PMC2860930
- DOI: 10.1172/JCI40145
The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice
Erratum in
- J Clin Invest. 2011 Apr 1;121(4):1668. Hatim, Hassan [corrected to Hassan, Hatim]
Abstract
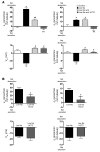
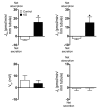
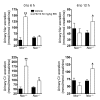
Regulation of sodium balance is a critical factor in the maintenance of euvolemia, and dysregulation of renal sodium excretion results in disorders of altered intravascular volume, such as hypertension. The amiloride-sensitive epithelial sodium channel (ENaC) is thought to be the only mechanism for sodium transport in the cortical collecting duct (CCD) of the kidney. However, it has been found that much of the sodium absorption in the CCD is actually amiloride insensitive and sensitive to thiazide diuretics, which also block the Na-Cl cotransporter (NCC) located in the distal convoluted tubule. In this study, we have demonstrated the presence of electroneutral, amiloride-resistant, thiazide-sensitive, transepithelial NaCl absorption in mouse CCDs, which persists even with genetic disruption of ENaC. Furthermore, hydrochlorothiazide (HCTZ) increased excretion of Na+ and Cl- in mice devoid of the thiazide target NCC, suggesting that an additional mechanism might account for this effect. Studies on isolated CCDs suggested that the parallel action of the Na+-driven Cl-/HCO3- exchanger (NDCBE/SLC4A8) and the Na+-independent Cl-/HCO3- exchanger (pendrin/SLC26A4) accounted for the electroneutral thiazide-sensitive sodium transport. Furthermore, genetic ablation of SLC4A8 abolished thiazide-sensitive NaCl transport in the CCD. These studies establish what we believe to be a novel role for NDCBE in mediating substantial Na+ reabsorption in the CCD and suggest a role for this transporter in the regulation of fluid homeostasis in mice.
Figures
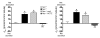

Similar articles
-
The Role of Intercalated Cell Nedd4-2 in BP Regulation, Ion Transport, and Transporter Expression.J Am Soc Nephrol. 2018 Jun;29(6):1706-1719. doi: 10.1681/ASN.2017080826. Epub 2018 May 17. J Am Soc Nephrol. 2018. PMID: 29773687 Free PMC article.
-
The chloride-bicarbonate exchanger pendrin is increased in the kidney of the pregnant rat.Exp Physiol. 2015 Oct;100(10):1177-86. doi: 10.1113/EP085396. Epub 2015 Sep 2. Exp Physiol. 2015. PMID: 26260990 Free PMC article.
-
Nitric oxide reduces Cl⁻ absorption in the mouse cortical collecting duct through an ENaC-dependent mechanism.Am J Physiol Renal Physiol. 2013 Jun 1;304(11):F1390-7. doi: 10.1152/ajprenal.00292.2012. Epub 2013 Mar 20. Am J Physiol Renal Physiol. 2013. PMID: 23515718 Free PMC article.
-
Novel mechanisms for NaCl reabsorption in the collecting duct.Curr Opin Nephrol Hypertens. 2011 Sep;20(5):506-11. doi: 10.1097/MNH.0b013e3283486c4a. Curr Opin Nephrol Hypertens. 2011. PMID: 21610493 Review.
-
Cortical distal nephron Cl(-) transport in volume homeostasis and blood pressure regulation.Am J Physiol Renal Physiol. 2013 Aug 15;305(4):F427-38. doi: 10.1152/ajprenal.00022.2013. Epub 2013 May 1. Am J Physiol Renal Physiol. 2013. PMID: 23637202 Free PMC article. Review.
Cited by
-
Renal intercalated cells are rather energized by a proton than a sodium pump.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7928-33. doi: 10.1073/pnas.1221496110. Epub 2013 Apr 22. Proc Natl Acad Sci U S A. 2013. PMID: 23610411 Free PMC article.
-
Overexpression of pendrin in intercalated cells produces chloride-sensitive hypertension.J Am Soc Nephrol. 2013 Jun;24(7):1104-13. doi: 10.1681/ASN.2012080787. Epub 2013 Jun 13. J Am Soc Nephrol. 2013. PMID: 23766534 Free PMC article.
-
Endothelin-1 inhibits sodium reabsorption by ET(A) and ET(B) receptors in the mouse cortical collecting duct.Am J Physiol Renal Physiol. 2013 Aug 15;305(4):F568-73. doi: 10.1152/ajprenal.00613.2012. Epub 2013 May 22. Am J Physiol Renal Physiol. 2013. PMID: 23698114 Free PMC article.
-
A mouse model for distal renal tubular acidosis reveals a previously unrecognized role of the V-ATPase a4 subunit in the proximal tubule.EMBO Mol Med. 2012 Oct;4(10):1057-71. doi: 10.1002/emmm.201201527. Epub 2012 Aug 30. EMBO Mol Med. 2012. PMID: 22933323 Free PMC article.
-
Mechanistic insights into the primary and secondary alterations of renal ion and water transport in the distal nephron.J Intern Med. 2023 Jan;293(1):4-22. doi: 10.1111/joim.13552. Epub 2022 Aug 21. J Intern Med. 2023. PMID: 35909256 Free PMC article. Review.
References
-
- Guyton AC. Blood pressure control — special role of the kidneys and body fluids. Science. 1991;252(5014):1813–1816. - PubMed
-
- Tse CM, Brant SR, Walker MS, Pouyssegur J, Donowitz M. Cloning and sequencing of a rabbit cDNA encoding an intestinal and kidney-specific Na+/H+ exchanger isoform (NHE-3). J Biol Chem. 1992;267(13):9340–9346. - PubMed
-
- Gamba G, et al. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem. 1994;269(26):17713–17722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

