Natural occurrence of 2',5'-linked heteronucleotides in marine sponges
- PMID: 20390103
- PMCID: PMC2852836
- DOI: 10.3390/md8020235
Natural occurrence of 2',5'-linked heteronucleotides in marine sponges
Abstract
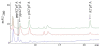
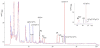
2',5'-oligoadenylate synthetases (OAS) as a component of mammalian interferon-induced antiviral enzymatic system catalyze the oligomerization of cellular ATP into 2',5'-linked oligoadenylates (2-5A). Though vertebrate OASs have been characterized as 2'-nucleotidyl transferases under in vitro conditions, the natural occurrence of 2',5'-oligonucleotides other than 2-5A has never been demonstrated. Here we have demonstrated that OASs from the marine sponges Thenea muricata and Chondrilla nucula are able to catalyze in vivo synthesis of 2-5A as well as the synthesis of a series 2',5'-linked heteronucleotides which accompanied high levels of 2',5'-diadenylates. In dephosphorylated perchloric acid extracts of the sponges, these heteronucleotides were identified as A2'p5'G, A2' p5'U, A2'p5'C, G2'p5'A and G2' p5'U. The natural occurrence of 2'-adenylated NAD(+) was also detected. In vitro assays demonstrated that besides ATP, GTP was a good substrate for the sponge OAS, especially for OAS from C. nucula. Pyrimidine nucleotides UTP and CTP were also used as substrates for oligomerization, giving 2',5'-linked homo-oligomers. These data refer to the substrate specificity of sponge OASs that is remarkably different from that of vertebrate OASs. Further studies of OASs from sponges may help to elucidate evolutionary and functional aspects of OASs as proteins of the nucleotidyltransferase family.
Keywords: 2′,5′-linked heteronucleotides; 2′,5′-oligoadenylate synthetase; OAS; marine sponge; natural products.
Figures
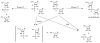
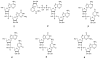

Similar articles
-
Enzymatically active 2',5'-oligoadenylate synthetases are widely distributed among Metazoa, including protostome lineage.Biochimie. 2014 Feb;97:200-9. doi: 10.1016/j.biochi.2013.10.015. Epub 2013 Oct 31. Biochimie. 2014. PMID: 24184688
-
2'-phosphodiesterase and 2',5'-oligoadenylate synthetase activities in the lowest metazoans, sponge [porifera].Biochimie. 2009 Nov-Dec;91(11-12):1531-4. doi: 10.1016/j.biochi.2009.07.015. Epub 2009 Aug 6. Biochimie. 2009. PMID: 19665065
-
Sponge OAS has a distinct genomic structure within the 2-5A synthetase family.Mol Genet Genomics. 2008 Nov;280(5):453-66. doi: 10.1007/s00438-008-0379-5. Epub 2008 Sep 17. Mol Genet Genomics. 2008. PMID: 18797928
-
The human 2',5'-oligoadenylate synthetase family: interferon-induced proteins with unique enzymatic properties.J Interferon Cytokine Res. 1999 Apr;19(4):295-308. doi: 10.1089/107999099313992. J Interferon Cytokine Res. 1999. PMID: 10334380 Review.
-
2-5A and virus infection.Prog Mol Subcell Biol. 1994;14:150-75. doi: 10.1007/978-3-642-78549-8_9. Prog Mol Subcell Biol. 1994. PMID: 8061884 Review. No abstract available.
References
-
- Hovanessian AG. On the discovery of interferon-inducible, double-stranded RNA activated enzymes: the 2′–5′oligoadenylate synthetases and the protein kinase PKR. Cytokine Growth Factor Rev. 2007;18:351–361. - PubMed
-
- Williams BR, Golgher RR, Kerr IM. Activation of a nuclease by pppA2′p5′A2′p5′A in intact cells. FEBS Lett. 1979;105:47–52. - PubMed
-
- Hovanessian AG, Wood JN. Anticellular and antiviral effects of pppA(2′p5′A)n. Virology. 1980;101:81–90. - PubMed
-
- Zhou A, Hassel BA, Silverman RH. Expression cloning of 2-5A-dependent RNAase: A uniquely regulated mediator of interferon action. Cell. 1993;72:753–765. - PubMed
-
- Kerr IM. The 2–5A system: A personal view. J Interferon Res. 1987;7:505–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
