Alkaloids in marine algae
- PMID: 20390105
- PMCID: PMC2852838
- DOI: 10.3390/md8020269
Alkaloids in marine algae
Abstract
This paper presents the alkaloids found in green, brown and red marine algae. Algal chemistry has interested many researchers in order to develop new drugs, as algae include compounds with functional groups which are characteristic from this particular source. Among these compounds, alkaloids present special interest because of their pharmacological activities. Alkaloid chemistry has been widely studied in terrestrial plants, but the number of studies in algae is insignificant. In this review, a detailed account of macro algae alkaloids with their structure and pharmacological activities is presented. The alkaloids found in marine algae may be divided into three groups: 1. Phenylethylamine alkaloids, 2. Indole and halogenated indole alkaloids, 3. Other alkaloids.
Keywords: alkaloids; halogenated indole alkaloids; indole alkaloids; other alkaloids; phenylethylamine alkaloids.
Figures

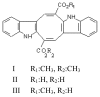










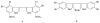


References
-
- Pelletier SW. Chemistry of the Alkaloids. Van Nostrand Reinhold; New York, NY, USA: 1970. p. 1.
-
- Trier G. Die Alkaloide. Verlag von Gebrüder; Borntraeger, Berlin, Germany: 1931. pp. 1–10.
-
- Swan GA. An Introduction to the Alkaloids. Black Well Scientific; Oxford and Edinburgh, UK: 1967. p. 1.
-
- Bentley KW. The Alkaloids. Interscience; New York, NY, USA: 1957. p. 1.
-
- Numata A, Takahashi C, Ito Y, Takada T, Kawai K, Usami Y, Matsumura E, Imachi M, Ito T, Hasegawo T. Communesin, cytotoxic metabolites of a fungus isolated from a marine algae. Tetrahedron Lett. 1993;34:2355–2358.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
