Role of protein kinase C in TBT-induced inhibition of lytic function and MAPK activation in human natural killer cells
- PMID: 20390410
- PMCID: PMC2909453
- DOI: 10.1007/s00244-010-9520-7
Role of protein kinase C in TBT-induced inhibition of lytic function and MAPK activation in human natural killer cells
Abstract
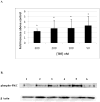
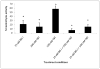
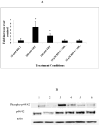
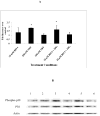
Human natural killer (NK) cells are lymphocytes that destroy tumor and virally infected cells. Previous studies have shown that exposure of NK cells to tributyltin (TBT) greatly diminishes their ability to destroy tumor cells (lytic function) while activating mitogen-activated protein kinases (MAPK) (p44/42, p38, and JNK) in NK cells. The signaling pathway that regulates NK lytic function appears to include activation of protein kinase C(PKC) as well as MAPK activity. TBT-induced activation of MAPKs would trigger a portion of the NK lytic signaling pathway, which would then leave the NK cell unable to trigger this pathway in response to a subsequent encounter with a target cell. In the present study we evaluated the involvement of PKC in inhibition of NK lysis of tumor cells and activation of MAPKs caused by TBT exposure. TBT caused a 2–3-fold activation of PKC at concentrations ranging from 50 to 300 nM (16–98 ng/ml),indicating that activation of PKC occurs in response to TBT exposure. This would then leave the NK cell unable to respond to targets. Treatment with the PKC inhibitor, bisindolylmaleimide I, caused an 85% decrease in the ability of NK cells to lyse tumor cells, validating the involvement of PKC in the lytic signaling pathway. The role of PKC in the activation of MAPKs by TBT was also investigated using bisindolylmaleimide I. The results indicated that, in NK cells where PKC activation was blocked, there was no activation of the MAPK, p44/42 in response to TBT.However, TBT-induced activation of the MAPKs, p38 and JNK did not require PKC activation. These results indicate the pivotal role of PKC in the TBT-induced loss of NK lytic function including activation of p44/42 by TBT in NK cells.
Figures

Similar articles
-
Activation of p44/42 MAPK plays a role in the TBT-induced loss of human natural killer (NK) cell function.Cell Biol Toxicol. 2010 Oct;26(5):435-44. doi: 10.1007/s10565-010-9154-6. Epub 2010 Mar 7. Cell Biol Toxicol. 2010. PMID: 20213532 Free PMC article.
-
Activation of protein kinase C and protein kinase D in human natural killer cells: effects of tributyltin, dibutyltin, and tetrabromobisphenol A.Toxicol Mech Methods. 2015;25(9):680-8. doi: 10.3109/15376516.2015.1070226. Epub 2015 Jul 31. Toxicol Mech Methods. 2015. PMID: 26228090 Free PMC article.
-
The role of p44/42 activation in tributyltin-induced inhibition of human natural killer cells: effects of MEK inhibitors.J Appl Toxicol. 2009 Mar;29(2):165-73. doi: 10.1002/jat.1397. J Appl Toxicol. 2009. PMID: 18989867 Free PMC article.
-
Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells.Toxicology. 2006 Jul 25;224(3):229-37. doi: 10.1016/j.tox.2006.05.002. Epub 2006 Jun 14. Toxicology. 2006. PMID: 16781040
-
Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells.Toxicology. 2005 May 5;209(3):263-77. doi: 10.1016/j.tox.2004.12.034. Toxicology. 2005. PMID: 15795062
Cited by
-
Endocrine Disruptor Compounds-A Cause of Impaired Immune Tolerance Driving Inflammatory Disorders of Pregnancy?Front Endocrinol (Lausanne). 2021 Apr 12;12:607539. doi: 10.3389/fendo.2021.607539. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33912131 Free PMC article. Review.
-
Kinome analysis of receptor-induced phosphorylation in human natural killer cells.PLoS One. 2012;7(1):e29672. doi: 10.1371/journal.pone.0029672. Epub 2012 Jan 4. PLoS One. 2012. PMID: 22238634 Free PMC article.
-
Tributyltin and dibutyltin alter secretion of tumor necrosis factor alpha from human natural killer cells and a mixture of T cells and natural killer cells.J Appl Toxicol. 2013 Jun;33(6):503-10. doi: 10.1002/jat.2822. Epub 2012 Oct 10. J Appl Toxicol. 2013. PMID: 23047847 Free PMC article.
-
Effects of butyltins on mitogen-activated-protein kinase kinase kinase and Ras activity in human natural killer cells.J Appl Toxicol. 2014 Sep;34(9):1002-11. doi: 10.1002/jat.2921. Epub 2013 Sep 5. J Appl Toxicol. 2014. PMID: 24038145 Free PMC article.
-
Secretion of interferon gamma from human immune cells is altered by exposure to tributyltin and dibutyltin.Environ Toxicol. 2015 May;30(5):559-71. doi: 10.1002/tox.21932. Epub 2013 Dec 20. Environ Toxicol. 2015. PMID: 24357260 Free PMC article.
References
-
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. - PubMed
-
- Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. - PubMed
-
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Arch Toxicology. 2007;81:271–277. - PubMed
-
- Baaijens PA. Health effect screening and biological monitoring for workers in organotin industries. Toxicology analytics of the tributyltins: the present F status, Proceedings of the ORTEPA Workshop; Berlin. 15–16 May; Vlissingen-Oost, The Netherlands: ORTEP-Association; 1986. pp. 191–208.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

