Promoting effect of Antrodia camphorata as an immunomodulating adjuvant on the antitumor efficacy of HER-2/neu DNA vaccine
- PMID: 20390417
- PMCID: PMC11030179
- DOI: 10.1007/s00262-010-0852-y
Promoting effect of Antrodia camphorata as an immunomodulating adjuvant on the antitumor efficacy of HER-2/neu DNA vaccine
Abstract
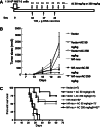
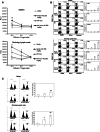
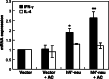
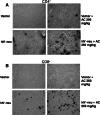
It is well known that DNA vaccines induce protective humoral and cell-mediated immune responses in several animal models. Antrodia camphorata (AC) is a unique basidiomycete fungus of the Polyporaceae family that only grows on the aromatic tree Cinnamomum kanehirai Hayata (Lauraceae) endemic to Taiwan. Importantly, AC has been shown to be highly beneficial in the treatment and prevention of cancer. The goal of this study was to investigate whether AC is able to augment the antitumor immune properties of a HER-2/neu DNA vaccine in a mouse model in which p185neu is overexpressed in MBT-2 tumor cells. Compared with the mice that received the HER-2/neu DNA vaccine alone, co-treatment with AC suppressed tumor growth and extended the survival rate. This increase in the antitumor efficacy was attributed to the enhancement of the Th1-like cellular immune response by the HER-2/neu DNA vaccine-AC combination. Evidence for this came from the marked increase in the IFN-gamma mRNA expression in CD4+ T cells in the draining inguinal lymph nodes, an increase in the number of functional HER-2/neu-specific CTLs, and the increased tumor infiltration of both CD4+ and CD8+ T cells, depletion of which abolishes the antitumor effect of the HER-2/neu DNA vaccine-AC therapy. Our results further indicate that the treatment of mice with AC enhanced DC activation and production of Th1-activating cytokines (e.g. IL-12, and IFN-alpha) in the draining lymph nodes, which were sufficient to directly stimulate T cell proliferation and higher IFN-gamma production in response to ErbB2. Overall, these results clearly demonstrate that AC represents a promising immunomodulatory adjuvant that could enhance the therapeutic potency of HER-2/neu DNA vaccines in cancer therapy.
Figures


Similar articles
-
A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells.Cancer Immunol Immunother. 2011 Jul;60(7):1019-27. doi: 10.1007/s00262-011-1016-4. Epub 2011 Apr 17. Cancer Immunol Immunother. 2011. PMID: 21499904 Free PMC article.
-
The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines.PLoS One. 2015 Feb 27;10(2):e0116191. doi: 10.1371/journal.pone.0116191. eCollection 2015. PLoS One. 2015. PMID: 25723174 Free PMC article.
-
The anti-cancer activity of Antrodia camphorata against human ovarian carcinoma (SKOV-3) cells via modulation of HER-2/neu signaling pathway.J Ethnopharmacol. 2013 Jun 21;148(1):254-65. doi: 10.1016/j.jep.2013.04.023. Epub 2013 Apr 22. J Ethnopharmacol. 2013. PMID: 23619020
-
Induction of antitumor immunity with combination of HER2/neu DNA vaccine and interleukin 2 gene-modified tumor vaccine.Clin Cancer Res. 2000 Nov;6(11):4381-8. Clin Cancer Res. 2000. PMID: 11106257
-
Unlocking the biological potential of methyl antcinate A: a new frontier in cancer and inflammation application.Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):4727-4745. doi: 10.1007/s00210-024-03544-3. Epub 2024 Dec 4. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39630281 Review.
Cited by
-
A novel adjuvant Ling Zhi-8 enhances the efficacy of DNA cancer vaccine by activating dendritic cells.Cancer Immunol Immunother. 2011 Jul;60(7):1019-27. doi: 10.1007/s00262-011-1016-4. Epub 2011 Apr 17. Cancer Immunol Immunother. 2011. PMID: 21499904 Free PMC article.
-
Emerging role of natural products in cancer immunotherapy.Acta Pharm Sin B. 2022 Mar;12(3):1163-1185. doi: 10.1016/j.apsb.2021.08.020. Epub 2021 Aug 21. Acta Pharm Sin B. 2022. PMID: 35530162 Free PMC article. Review.
-
Clitocybe nuda Activates Dendritic Cells and Acts as a DNA Vaccine Adjuvant.Evid Based Complement Alternat Med. 2013;2013:761454. doi: 10.1155/2013/761454. Epub 2013 Aug 22. Evid Based Complement Alternat Med. 2013. PMID: 24058377 Free PMC article.
-
Unleashing the therapeutic potential of tumor-draining lymph nodes: spotlight on bladder cancer.J Transl Med. 2025 Apr 29;23(1):489. doi: 10.1186/s12967-024-05864-7. J Transl Med. 2025. PMID: 40301883 Free PMC article. Review.
-
The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines.PLoS One. 2015 Feb 27;10(2):e0116191. doi: 10.1371/journal.pone.0116191. eCollection 2015. PLoS One. 2015. PMID: 25723174 Free PMC article.
References
-
- Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C, Lollini PL, Musiani P, Forni G, Cavallo F. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.CAN-03-2962. - DOI - PubMed
-
- Lu Y, Wei YQ, Tian L, Zhao X, Yang L, Hu B, Kan B, Wen YJ, Liu F, Deng HX, Li J, Mao YQ, Lei S, Huang MJ, Peng F, Jiang Y, Zhou H, Zhou LQ, Luo F. Immunogene therapy of tumors with vaccine based on xenogeneic epidermal growth factor receptor. J Immunol. 2003;170:3162–3170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

