Health-related quality of life in pain-free or mildly symptomatic patients with metastatic hormone-resistant prostate cancer following treatment with the specific endothelin A receptor antagonist zibotentan (ZD4054)
- PMID: 20390429
- PMCID: PMC11827807
- DOI: 10.1007/s00432-010-0864-1
Health-related quality of life in pain-free or mildly symptomatic patients with metastatic hormone-resistant prostate cancer following treatment with the specific endothelin A receptor antagonist zibotentan (ZD4054)
Abstract
Purpose: Zibotentan (ZD4054) is a specific endothelin A receptor antagonist in clinical development for the treatment of hormone-resistant prostate cancer (HRPC). In a Phase II trial in patients with pain-free or mildly symptomatic metastatic HRPC, zibotentan was well tolerated with a promising signal for prolonged overall survival compared with placebo. As part of this trial, the impact of zibotentan compared with placebo on health-related quality of life (HRQoL) was assessed.
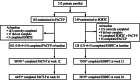
Methods: Patients were randomized to receive once-daily oral zibotentan 10 or 15 mg, or matching placebo. Patients were allocated to one of two questionnaires; the Functional Assessment of Cancer Therapy-Prostate (FACT-P) or the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), supplemented by PR25, specific for prostate cancer. Questionnaires were completed at baseline and every 4 weeks until disease progression when study treatment was discontinued.
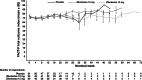
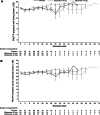
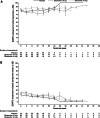
Results: Compliance with questionnaire completion was >90% (286 of 312 patients) of the intention-to-treat population at baseline. Of baseline completers who were available for assessment (i.e., had not clinically progressed), 89% (164 of 184) and 83% (73 of 88) completed questionnaires at 12 and 24 weeks, respectively. HRQoL scores from both questionnaires were high at baseline and remained high throughout the study, with scores being similar in the zibotentan and placebo groups. However, some floor and ceiling effects were seen in the EORTC QLQ-C30 questionnaire.
Conclusions: High-baseline HRQoL scores were maintained throughout treatment with zibotentan. The FACT-P instrument was selected to further assess the impact of zibotentan on HRQoL in the Phase III clinical trial program.
Keywords: EORTC QLQ-C30; FACT-P; Health-related quality of life (HRQoL); Hormone-resistant prostate cancer (HRPC); Zibotentan.
Figures
References
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376 - PubMed
-
- Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock IF, On behalf of the TAX-327 investigators (2008) Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res 14:2763–2767 - PubMed
-
- Borghede G, Sullivan M (1996) Measurement of quality of life in localized prostatic cancer patients treated with radiotherapy. Development of a prostate cancer-specific module supplementing the EORTC QLQ-C30. Qual Life Res 5:212–222 - PubMed
-
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579 - PubMed
-
- Cella D, Nichol MB, Eton D, Nelson J, Mulani P (2008) Estimating clinically meaningful changes for the functional assessment of cancer therapy-prostate: results from a clinical trial of patients with metastatic hormone-refractory prostate cancer. Value Health 12:124–129 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

