Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis
- PMID: 20390447
- PMCID: PMC2911540
- DOI: 10.1007/s10456-010-9165-1
Paramagnetic and fluorescent liposomes for target-specific imaging and therapy of tumor angiogenesis
Abstract
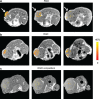
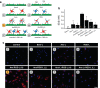
Angiogenesis is essential for tumor growth and metastatic potential and for that reason considered an important target for tumor treatment. Noninvasive imaging technologies, capable of visualizing tumor angiogenesis and evaluating the efficacy of angiostatic therapies, are therefore becoming increasingly important. Among the various imaging modalities, magnetic resonance imaging (MRI) is characterized by a superb spatial resolution and anatomical soft-tissue contrast. Revolutionary advances in contrast agent chemistry have delivered versatile angiogenesis-specific molecular MRI contrast agents. In this paper, we review recent advances in the preclinical application of paramagnetic and fluorescent liposomes for noninvasive visualization of the molecular processes involved in tumor angiogenesis. This liposomal contrast agent platform can be prepared with a high payload of contrast generating material, thereby facilitating its detection, and is equipped with one or more types of targeting ligands for binding to specific molecules expressed at the angiogenic site. Multimodal liposomes endowed with contrast material for complementary imaging technologies, e.g., MRI and optical, can be exploited to gain important preclinical insights into the mechanisms of binding and accumulation at angiogenic vascular endothelium and to corroborate the in vivo findings. Interestingly, liposomes can be designed to contain angiostatic therapeutics, allowing for image-supervised drug delivery and subsequent monitoring of therapeutic efficacy.
Figures
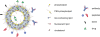



References
-
- Beliveau R, Gingras D, Kruger EA, et al. The antiangiogenic agent neovastat (AE-941) inhibits vascular endothelial growth factor-mediated biological effects. Clin Cancer Res. 2002;8:1242–1250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

