Activation and deactivation of DNAzyme and antisense function with light for the photochemical regulation of gene expression in mammalian cells
- PMID: 20392038
- PMCID: PMC2862549
- DOI: 10.1021/ja100710j
Activation and deactivation of DNAzyme and antisense function with light for the photochemical regulation of gene expression in mammalian cells
Abstract
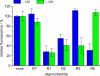
The photochemical regulation of biological systems represents a very precise means of achieving high-resolution control over gene expression in both a spatial and a temporal fashion. DNAzymes are enzymatically active deoxyoligonucleotides that enable the site-specific cleavage of RNA and have been used in a variety of in vitro applications. We have previously reported the photochemical activation of DNAzymes and antisense agents through the preparation of a caged DNA phosphoramidite and its site-specific incorporation into oligonucleotides. The presence of the caging group disrupts either DNA:RNA hybridization or catalytic activity until removed via a brief irradiation with UV light. Here, we are expanding this concept by investigating the photochemical deactivation of DNAzymes and antisense agents. Moreover, we report the application of light-activated and light-deactivated antisense agents to the regulation of gene function in mammalian cells. This represents the first example of gene silencing antisense agents that can be turned on and turned off in mammalian tissue culture.
Figures
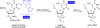









Similar articles
-
Optochemical control of deoxyoligonucleotide function via a nucleobase-caging approach.Acc Chem Res. 2014 Jan 21;47(1):45-55. doi: 10.1021/ar400036a. Epub 2013 Aug 28. Acc Chem Res. 2014. PMID: 23981235 Free PMC article.
-
Regulation of transcription through light-activation and light-deactivation of triplex-forming oligonucleotides in mammalian cells.ACS Chem Biol. 2012 Jul 20;7(7):1247-56. doi: 10.1021/cb300161r. Epub 2012 May 11. ACS Chem Biol. 2012. PMID: 22540192 Free PMC article.
-
Improved RNA cleavage by LNAzyme derivatives of DNAzymes.Biochem Soc Trans. 2004 Feb;32(Pt 1):37-40. doi: 10.1042/bst0320037. Biochem Soc Trans. 2004. PMID: 14748708
-
Suppression of gene expression by targeted disruption of messenger RNA: available options and current strategies.Stem Cells. 2000;18(5):307-19. doi: 10.1634/stemcells.18-5-307. Stem Cells. 2000. PMID: 11007915 Review.
-
DNA Catalysis: The Chemical Repertoire of DNAzymes.Molecules. 2015 Nov 20;20(11):20777-804. doi: 10.3390/molecules201119730. Molecules. 2015. PMID: 26610449 Free PMC article. Review.
Cited by
-
Catalytic DNA: Scope, Applications, and Biochemistry of Deoxyribozymes.Trends Biochem Sci. 2016 Jul;41(7):595-609. doi: 10.1016/j.tibs.2016.04.010. Epub 2016 May 25. Trends Biochem Sci. 2016. PMID: 27236301 Free PMC article. Review.
-
Metal-Dependent DNAzymes for the Quantitative Detection of Metal Ions in Living Cells: Recent Progress, Current Challenges, and Latest Results on FRET Ratiometric Sensors.Inorg Chem. 2019 Oct 21;58(20):13696-13708. doi: 10.1021/acs.inorgchem.9b01280. Epub 2019 Jul 31. Inorg Chem. 2019. PMID: 31364355 Free PMC article. Review.
-
Photochemical modifications for DNA/RNA oligonucleotides.RSC Adv. 2022 Feb 24;12(11):6484-6507. doi: 10.1039/d1ra05951c. eCollection 2022 Feb 22. RSC Adv. 2022. PMID: 35424630 Free PMC article. Review.
-
Mismatch Discrimination and Efficient Photomodulation with Split 10-23 DNAzymes.Inorganica Chim Acta. 2012 Jan 15;380:386-391. doi: 10.1016/j.ica.2011.10.068. Inorganica Chim Acta. 2012. PMID: 22544974 Free PMC article.
-
Precision Control of Light-Responsive Nucleic Acids Modified with Photoremovable Protecting Groups for Functionalization.JACS Au. 2025 Jun 19;5(7):2953-2976. doi: 10.1021/jacsau.5c00524. eCollection 2025 Jul 28. JACS Au. 2025. PMID: 40747049 Free PMC article. Review.
References
-
- Deiters A. Chembiochem. 2010;11:47. - PMC - PubMed
- Deiters A. Curr. Opin. Chem. Biol. 2009;13:678. - PMC - PubMed
- Lee HM, Larson DR, Lawrence DS. ACS Chem. Biol. 2009;4:409. - PMC - PubMed
- Young DD, Deiters A. Org. Biomol. Chem. 2007;5:999. - PubMed
- Curley K, Lawrence DS. Curr. Opin. Chem. Biol. 1999;3:84. - PubMed
-
- Adams SR, Tsien RY. Annu. Rev. Physiol. 1993;55:755. - PubMed
-
- Pelliccioli AP, Wirz J. Photochem. Photobiol. Sci. 2002;1:441. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

