Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates
- PMID: 20392112
- PMCID: PMC2887675
- DOI: 10.1021/cb1000313
Mutation of archaeal isopentenyl phosphate kinase highlights mechanism and guides phosphorylation of additional isoprenoid monophosphates
Abstract
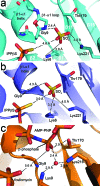
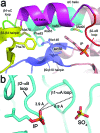
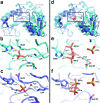
The biosynthesis of isopentenyl diphosphate (IPP) from either the mevalonate (MVA) or the 1-deoxy-d-xylulose 5-phosphate (DXP) pathway provides the key metabolite for primary and secondary isoprenoid biosynthesis. Isoprenoid metabolism plays crucial roles in membrane stability, steroid biosynthesis, vitamin production, protein localization, defense and communication, photoprotection, sugar transport, and glycoprotein biosynthesis. Recently, an alternative branch of the MVA pathway was discovered in the archaeon Methanocaldococcus jannaschii involving a small molecule kinase, isopentenyl phosphate kinase (IPK). IPK belongs to the amino acid kinase (AAK) superfamily. In vitro, IPK phosphorylates isopentenyl monophosphate (IP) in an ATP and Mg(2+)-dependent reaction producing IPP. Here, we describe crystal structures of IPK from M. jannaschii refined to nominal resolutions of 2.0-2.8 A. Notably, an active site histidine residue (His60) forms a hydrogen bond with the terminal phosphate of both substrate and product. This His residue serves as a marker for a subset of the AAK family that catalyzes phosphorylation of phosphate or phosphonate functional groups; the larger family includes carboxyl-directed kinases, which lack this active site residue. Using steady-state kinetic analysis of H60A, H60N, and H60Q mutants, the protonated form of the Nepsilon(2) nitrogen of His60 was shown to be essential for catalysis, most likely through hydrogen bond stabilization of the transition state accompanying transphosphorylation. Moreover, the structures served as the starting point for the engineering of IPK mutants capable of the chemoenzymatic synthesis of longer chain isoprenoid diphosphates from monophosphate precursors.
Figures



Similar articles
-
Characterization of thermophilic archaeal isopentenyl phosphate kinases.Biochemistry. 2010 Jan 12;49(1):207-17. doi: 10.1021/bi9017957. Biochemistry. 2010. PMID: 19928876 Free PMC article.
-
X-ray structures of isopentenyl phosphate kinase.ACS Chem Biol. 2010 May 21;5(5):517-27. doi: 10.1021/cb100032g. ACS Chem Biol. 2010. PMID: 20402538 Free PMC article.
-
Mutagenesis of isopentenyl phosphate kinase to enhance geranyl phosphate kinase activity.ACS Chem Biol. 2012 Jul 20;7(7):1241-6. doi: 10.1021/cb300106e. Epub 2012 May 10. ACS Chem Biol. 2012. PMID: 22533411 Free PMC article.
-
Isoprenoid biosynthesis via 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DOXP/MEP) pathway.Acta Biochim Pol. 2001;48(3):663-72. Acta Biochim Pol. 2001. PMID: 11833775 Review.
-
Isopentenyl diphosphate isomerase: A checkpoint to isoprenoid biosynthesis.Biochimie. 2012 Aug;94(8):1621-34. doi: 10.1016/j.biochi.2012.03.021. Epub 2012 Apr 4. Biochimie. 2012. PMID: 22503704 Review.
Cited by
-
Harnessing evolutionary diversification of primary metabolism for plant synthetic biology.J Biol Chem. 2019 Nov 8;294(45):16549-16566. doi: 10.1074/jbc.REV119.006132. Epub 2019 Sep 26. J Biol Chem. 2019. PMID: 31558606 Free PMC article. Review.
-
Functional Characterization and Screening of Promiscuous Kinases and Isopentenyl Phosphate Kinases for the Synthesis of DMAPP via a One-Pot Enzymatic Cascade.Int J Mol Sci. 2022 Oct 26;23(21):12904. doi: 10.3390/ijms232112904. Int J Mol Sci. 2022. PMID: 36361694 Free PMC article.
-
Probing the Substrate Promiscuity of Isopentenyl Phosphate Kinase as a Platform for Hemiterpene Analogue Production.Chembiochem. 2019 Sep 2;20(17):2217-2221. doi: 10.1002/cbic.201900135. Epub 2019 Jul 22. Chembiochem. 2019. PMID: 30998839 Free PMC article.
-
The Streptomyces-produced antibiotic fosfomycin is a promiscuous substrate for archaeal isopentenyl phosphate kinase.Biochemistry. 2012 Jan 31;51(4):917-25. doi: 10.1021/bi201662k. Epub 2012 Jan 11. Biochemistry. 2012. PMID: 22148590 Free PMC article.
-
Improving the catalytic activity of isopentenyl phosphate kinase through protein coevolution analysis.Sci Rep. 2016 Apr 7;6:24117. doi: 10.1038/srep24117. Sci Rep. 2016. PMID: 27052337 Free PMC article.
References
-
- Gershenzon J.; Dudareva N. (2007) The Function of Terpene Natural Products in the Natural World. Nat. Chem. Biol. 3, 408–414. - PubMed
-
- Novakova Z.; Surin S.; Blasko J.; Majernik A.; Smigan P. (2008) Membrane Proteins and Squalene-Hydrosqualene Profile in Methanoarchaeon Methanothermobacter Thermautotrophicus Resistant to N, N′-Dicyclohexylcarbodiimide. Folia Microbiol. (Prague, Czech Repub.) 53, 237–240. - PubMed
-
- Ourisson G.; Rohmer M.; Poralla K. (1987) Prokaryotic Hopanoids and Other Polyterpenoid Sterol Surrogates. Annu. Rev. Microbiol. 41, 301–333. - PubMed
-
- Sieiro C.; Poza M.; de Miguel T.; Villa T. G. (2003) Genetic Basis of Microbial Carotenogenesis. Int. Microbiol. 6, 11–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

