Lanthanum tricyanide-catalyzed acyl silane-ketone benzoin additions and kinetic resolution of resultant alpha-silyloxyketones
- PMID: 20392127
- PMCID: PMC2873159
- DOI: 10.1021/jo100312w
Lanthanum tricyanide-catalyzed acyl silane-ketone benzoin additions and kinetic resolution of resultant alpha-silyloxyketones
Abstract
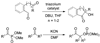
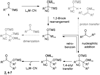
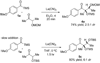
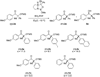
We report the full account of our efforts on the lanthanum tricyanide-catalyzed acyl silane-ketone benzoin reaction. The reaction exhibits a wide scope in both acyl silane (aryl, alkyl) and ketone (aryl-alkyl, alkyl-alkyl, aryl-aryl, alkenyl-alkyl, alkynyl-alkyl) coupling partners. The diastereoselectivity of the reaction has been examined in both cyclic and acyclic systems. Cyclohexanones give products arising from equatorial attack by the acyl silane. The diastereoselectivity of acyl silane addition to acyclic alpha-hydroxy ketones can be controlled by varying the protecting group to obtain either Felkin-Ahn or chelation control. The resultant alpha-silyloxyketone products can be resolved with selectivity factors from 10 to 15 by subjecting racemic ketone benzoin products to CBS reduction.
Figures
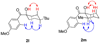
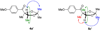



References
-
- Corey EJ, Seebach D. Angew. Chem. Int. Ed. 1965;4:1077–1078.
-
- Lapworth A. J. Chem. Soc. 1903;83:995–1005.
-
- Enders D, Kalfass U. Angew. Chem., Int. Ed. 2002;41:1743–1745. and references therein. - PubMed
- Dunkelmann P, Kolter-Jung D, Nitsche A, Demir AS, Siegert P, Lingen B, Baumann M, Pohl M, Muller M. J. Am. Chem. Soc. 2002;124:12084–12085. - PubMed
- Hachisu Y, Bode JW, Suzuki K. Adv. Synth. Catal. 2004;346:1097–1100.
- Enders D, Oliver N. Synlet. 2004:2111–2114.
-
- Linghu X, Potnick JR, Johnson JS. J. Am. Chem. Soc. 2004;126:3070–3071. - PubMed
-
-
Deprotonation of silyl cyanohydrins: Bunnelle EM, Smith CR, Lee SK, Singaram SW, Rhodes AJ, Sarpong R. Tetrahedron. 2008;64:7008–7014. Wessig P, Glombitza C, Mueller G, Teubner J. J. Org. Chem. 2004;69:7582–7591. Lithiation of vinyl ethers: Palomo C, Oiarbide M, Arceo E, Garcia JM, Lopez R, Gonzalez A, Linden A. Angew. Chem., Int. Ed. 2005;44:6187–6190. Deprotonation of dithianes: Nicolaou KC, Magolda RL, Sipio WJ, Barnette WE, Lysenko Z, Joullie MM. J. Am. Chem. Soc. 1980;102:3784–3793.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

