Dynamics of the transition from osteoblast to osteocyte
- PMID: 20392270
- PMCID: PMC2981593
- DOI: 10.1111/j.1749-6632.2009.05246.x
Dynamics of the transition from osteoblast to osteocyte
Abstract
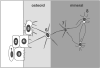
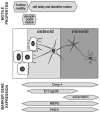
Osteocytes are derived from osteoblasts and make up over 90% of the cells in bone. However, the mechanisms that control the differentiation of osteoblasts into osteocytes embedded in bone matrix are not well understood. With the recent developments of transgenic models for manipulating gene expression in osteocytes and of transgenic mice carrying lineage reporters for osteoblasts and osteocytes, unprecedented new insights are becoming possible. In this article we review recent advances, such as comparative gene and protein expression studies, that are delineating the changes in gene and protein expression that accompany osteocyte differentiation. We also review recent studies in which time-lapse dynamic imaging approaches have been used to visualize osteoblast and osteocyte populations within bone. These approaches reveal the key role of cell motility in bone cell function and highlight the dynamic nature of mineralized tissues. Changes in motile properties of the cell may be key in the transition from osteoblast to osteocyte, as reflected in the altered expression of many molecules involved in cytoskeletal function.
Figures
References
-
- Gegenbauer C. Carpus und Tarsus. Leipzig; Schultergtel: 1864. Untersuchungen zur vergleichenden Anatomie der Wirbeltiere. Heft 1.
-
- Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235:176–90. - PubMed
-
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. - PubMed
-
- Jilka RL, et al. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. - PubMed
-
- Li M, et al. Histochemical evidence of the initial chondrogenesis and osteogenesis in the periosteum of a rib fractured model: implications of osteocyte involvement in periosteal chondrogenesis. Microsc Res Tech. 2004;64:330–42. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

