Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission
- PMID: 20392301
- PMCID: PMC2990954
- DOI: 10.1017/S0952523810000076
Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission
Abstract
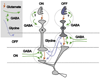
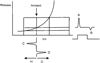
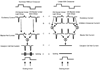
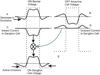
Early retinal studies categorized ganglion cell behavior as either linear or nonlinear and rectifying as represented by the familiar X- and Y-type ganglion cells in cat. Nonlinear behavior is in large part a consequence of the rectifying nonlinearities inherent in synaptic transmission. These nonlinear signals underlie many special functions in retinal processing, including motion detection, motion in motion, and local edge detection. But linear behavior is also required for some visual processing tasks. For these tasks, the inherently nonlinear signals are "linearized" by "crossover inhibition." Linearization utilizes a circuitry whereby nonlinear ON inhibition adds with nonlinear OFF excitation or ON excitation adds with OFF inhibition to generate a more linear postsynaptic voltage response. Crossover inhibition has now been measured in most bipolar, amacrine, and ganglion cells. Functionally crossover inhibition enhances edge detection, allows ganglion cells to recognize luminance-neutral patterns with their receptive fields, permits ganglion cells to distinguish contrast from luminance, and maintains a more constant conductance during the light response. In some cases, crossover extends the operating range of cone-driven OFF ganglion cells into the scotopic levels. Crossover inhibition is also found in neurons of the lateral geniculate nucleus and V1.
Figures





References
-
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. Journal of Neurophysiology. 2000;84:909–926. - PubMed
-
- Arkin MS, Miller RF. Bipolar origin of synaptic inputs to sustained OFF-ganglion cells in the mudpuppy retina. Journal of Neurophysiology. 1988;60:1122–1142. - PubMed
-
- Belgum JH, Dvorak DR, McReynolds JS. Light-evoked sustained inhibition in mudpuppy retinal ganglion cells. Vision Research. 1982;22:257–260. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

