Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin
- PMID: 20392471
- PMCID: PMC3401642
- DOI: 10.1016/j.virol.2010.03.021
Mannose-rich glycosylation patterns on HIV-1 subtype C gp120 and sensitivity to the lectins, Griffithsin, Cyanovirin-N and Scytovirin
Abstract
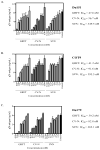
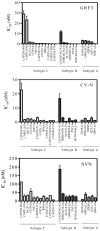
Griffithsin (GRFT), Cyanovirin-N (CV-N) and Scytovirin (SVN) are lectins that inhibit HIV-1 infection by binding to multiple mannose-rich glycans on the HIV-1 envelope glycoproteins (Env). Here we show that these lectins neutralize subtype C primary virus isolates in addition to Env-pseudotyped viruses obtained from plasma and cervical vaginal lavages. Among 15 subtype C pseudoviruses, the median IC(50) values were 0.4, 1.8 and 20.1nM for GRFT, CV-N and SVN, respectively, similar to what was found for subtype B and A. Analysis of Env sequences suggested that concomitant lack of glycans at positions 234 and 295 resulted in natural resistance to these compounds, which was confirmed by site-directed mutagenesis. Furthermore, the binding sites for these lectins overlapped that of the 2G12 monoclonal antibody epitope, which is generally absent on subtype C Env. This data support further research on these lectins as potential microbicides in the context of HIV-1 subtype C infection.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Balzarini J. Targeting the glycans of gp120: a novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5(11):726–31. - PubMed
-
- Balzarini J, Van Damme L. Microbicide drug candidates to prevent HIV infection. Lancet. 2007;369:787–97. - PubMed
-
- Balzarini J, Van Laethem K, Peumans WJ, Van Damme EJ, Bolmstedt A, Gago F, Schols D. Mutational pathways, resistance profile, and side effects of cyanovirin relative to human immunodeficiency virus type 1 strains with N-glycan deletions in their gp120 envelopes. J Virol. 2006;80(17):8411–21. - PMC - PubMed
-
- Barrientos LG, Louis JM, Botos I, Mori T, Han Z, O’Keefe BR, Boyd MR, Wlodawer A, Gronenborn AM. The domain-swapped dimer of cyanovirin-N is in a metastable folded state: reconciliation of X-ray and NMR structures. Structure. 2002;10(5):673–86. - PubMed
-
- Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78(23):13232–52. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

