AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress
- PMID: 20392689
- PMCID: PMC2888409
- DOI: 10.1074/jbc.M110.102467
AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress
Erratum in
-
Correction: AMPKα1 deletion shortens erythrocyte life span in mice: Role of oxidative stress.J Biol Chem. 2019 Jul 5;294(27):10738. doi: 10.1074/jbc.AAC119.009647. J Biol Chem. 2019. PMID: 31278157 Free PMC article. No abstract available.
Abstract
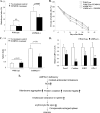
AMP-activated protein kinase (AMPK) is an energy sensor essential for maintaining cellular energy homeostasis. Here, we report that AMPKalpha1 is the predominant isoform of AMPK in murine erythrocytes and mice globally deficient in AMPKalpha1 (AMPKalpha1(-/-)), but not in those lacking AMPKalpha2, and the mice had markedly enlarged spleens with dramatically increased proportions of Ter119-positive erythroid cells. Blood tests revealed significantly decreased erythrocyte and hemoglobin levels with increased reticulocyte counts and elevated plasma erythropoietin concentrations in AMPKalpha1(-/-) mice. The life span of erythrocytes from AMPKalpha1(-/-) mice was less than that in wild-type littermates, and the levels of reactive oxygen species and oxidized proteins were significantly increased in AMPKalpha1(-/-) erythrocytes. In keeping with the elevated oxidative stress, treatment of AMPKalpha1(-/-) mice with the antioxidant, tempol, resulted in decreased reticulocyte counts and improved erythrocyte survival. Furthermore, the expression of Foxo3 and reactive oxygen species scavenging enzymes was significantly decreased in erythroblasts from AMPKalpha1(-/-) mice. Collectively, these results establish an essential role for AMPKalpha1 in regulating oxidative stress and life span in erythrocytes.
Figures



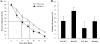
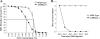

References
-
- Zou M. H. (2007) Prostaglandins Other Lipid Mediat. 82, 119–127 - PubMed
-
- Castro L., Freeman B. A. (2001) Nutrition 17, 161–165 - PubMed
-
- Johnson R. M., Goyette G., Jr., Ravindranath Y., Ho Y. S. (2005) Free Radic. Biol. Med. 39, 1407–1417 - PubMed
-
- Neumann C. A., Krause D. S., Carman C. V., Das S., Dubey D. P., Abraham J. L., Bronson R. T., Fujiwara Y., Orkin S. H., Van Etten R. A. (2003) Nature 424, 561–565 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

